Solid Renal Masses
Rinze Reinhard, Mandy van der Zon-Conijn and Robin Smithuis
Radiology department of the Onze Lieve Vrouwe Gasthuis in Amsterdam, Medical Center Haaglanden-Bronovo in the Hague, Leiden University Medical Center in Leiden and the Alrijne hospital in Leiderdorp, the Netherlands
Publicationdate
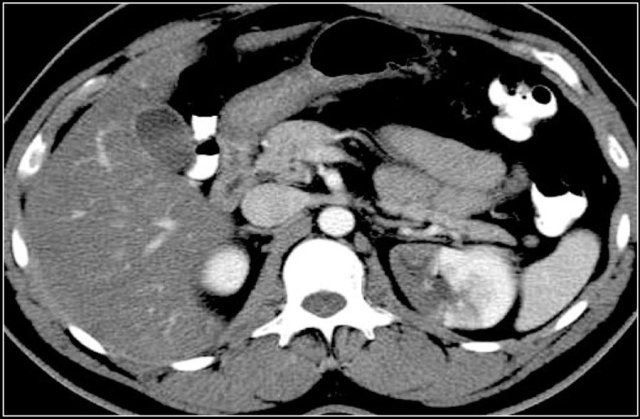
Most renal masses are incidental findings.
Many of these masses are renal cell carcinomas.
The goal of imaging is to differentiate these renal cell carcinomas from benign disease, although in many cases it may not be possible.
There are certain imaging findings, however, which are in favor of benign disease or low grade malignancy.
These imaging findings may offer guidance to patients and referring physicians in making management decisions. Options include partial or radical nephrectomy, biopsy and tumor ablation or follow up with watchful waiting.
In this article we will discuss imaging features of benign and malignant renal tumors and tumor mimics.
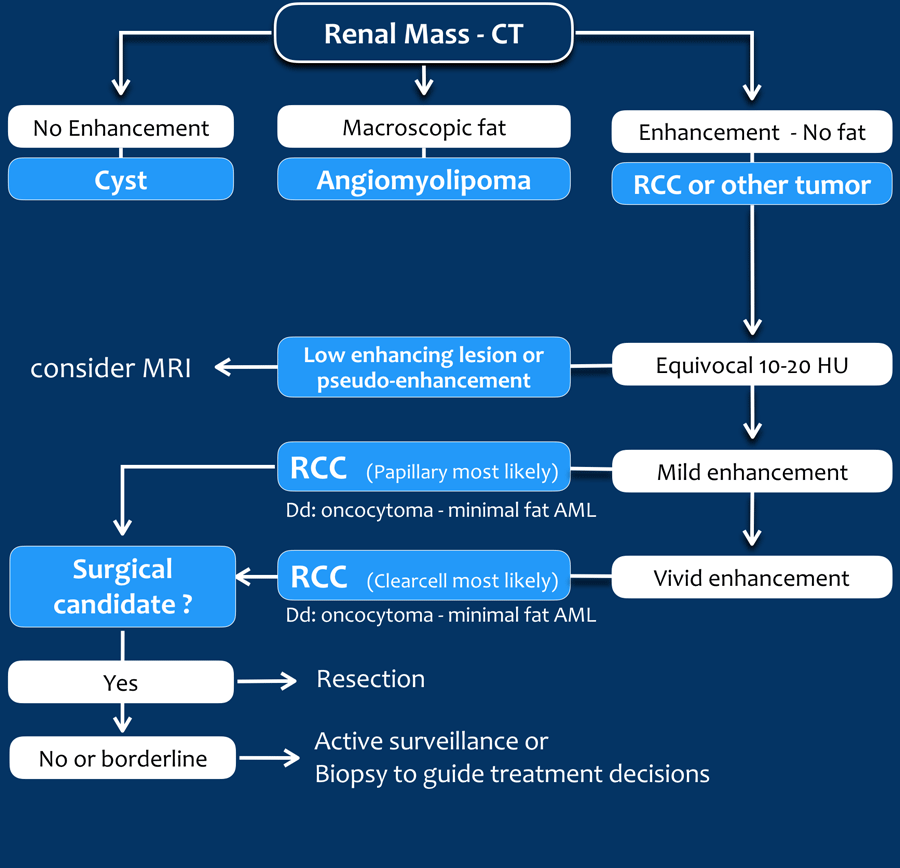
Differentiation of renal masses
The steps in the differentiation of a renal lesion are:
- First determine whether the lesion is a cyst.
- If it is not a cyst, look for macroscopic fat, which means that it is a benign angiomyolipoma (AML).
- Exclude tumor-mimics like infection and infarction, which usually present in different clinical settings.
- Exclude metastatic disease and lymphoma; kidney localizations are usually only seen in widespread disease.
Once you have followed these steps, there will be many cases in which a final diagnosis can not be made and renal cell carcinoma is still on top of your list of differential diagnoses.
Use CT and MR to look for findings that are in favor of a benign or low-grade tumor versus a high-grade renal cell carcinoma.
CT
Hyperdensity on unenhanced CT
A lesion with a density > 70 HU on an unenhanced CT scan is a hemorrhagic cyst.
Hemorrhagic cysts may have densities lower than 70 HU, but in these cases we also need to check the post-contrast series for any enhancement.
Lack of enhancement confirms the cystic nature of these lesions.
Macroscopic fat < -20 HU in a renal mass is a reliable sign of an angiomyolipoma.
Thin slices can be helpful to determine the density.
Unfortunately 5% of AMLs do not contain macroscopic fat.
These lipid-poor AMLs can not be distinguished from renal cell carcinoma.
In rare cases, RCCs can also contain fat.
The presence of calcifications and fat should raise the suspicion of a RCC.
Equivocal enhancement of 10-20 HU can be due to pseudo-enhancement in a cyst as a result of beam hardening.
MRI can be helpful in differentiating these cases, since it better depicts enhancement.
Equivocal enhancement can also be seen in low-enhancing lesions like papillary renal cell carcinoma, which usually is a less aggressive subtype than the more common clear cell carcinoma.
Homogeneous enhancement and a high attenuation value on unenhanced CT (> 40 HU) is in favor of the diagnosis of a lipid-poor AML, although RCC can not be reliably excluded.
Strong enhancement is seen in clear cell carcinoma, lipid-poor AML and oncocytoma.
Since clear cell carcinoma is far more common than an oncocytoma or a lipid-poor AML, clear cell carcinoma is the most likely diagnosis, especially in a large and heterogeneous mass.
Mention the possibility of a lipid-poor AML or oncocytoma in your report, as surveillance or biopsy may be an option in poor surgical candidates.
MRI
High signal on T1-weighted images is typically seen in hemorrhagic or proteinaceous cysts and in angiomyolipomas that contain macroscopic extracellular fat.
Intracellular fat however does not result in a high signal on T1-weighted images but it results in a signal drop on out of phase images.
This can be seen in minimal fat AML or RCC.
82% of clear cell RCC have intracellular fat, which has a 90% specificity for the diagnosis of a clear cell renal cell carcinoma.
MRI is better than CT in the accurate diagnosis of a cystic lesion and it can better depict enhancement and differentiate CT-pseudo-enhancement from real enhancement.
Low T2-signal is in favor of papillary RCC or minimal fat angiomyolipoma.
High T2 is typically seen in clear cell RCC but is not specific, since it can also be seen in oncocytomas.
So again there is much overlap between benign and malignant tumors.
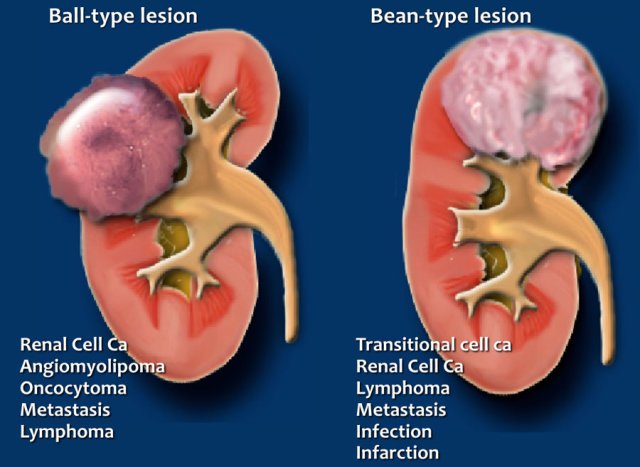
Ball or Bean
Another way to look at renal solid masses is to look at the shape.
Solid lesions can be divided into ball-type and bean-type lesions.
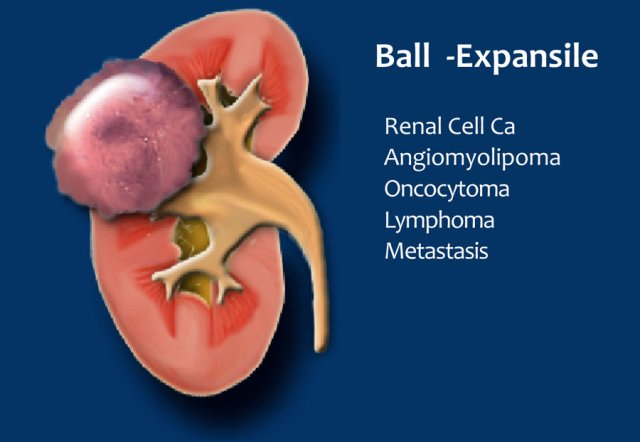
Ball-type lesions are the most common and present as expansile masses, deforming the renal contour.
Renal cell carcinomas and oncocytomas are typical ball-type lesions.
Bean-type lesions do not deform the renal contour and the bean-shape of the kidney is preserved.
Bean-type lesions are more difficult to detect and usually not visible on unenhanced CT images.
Notice that there is much overlap in the differential diagnosis of ball-type and bean-type lesions.
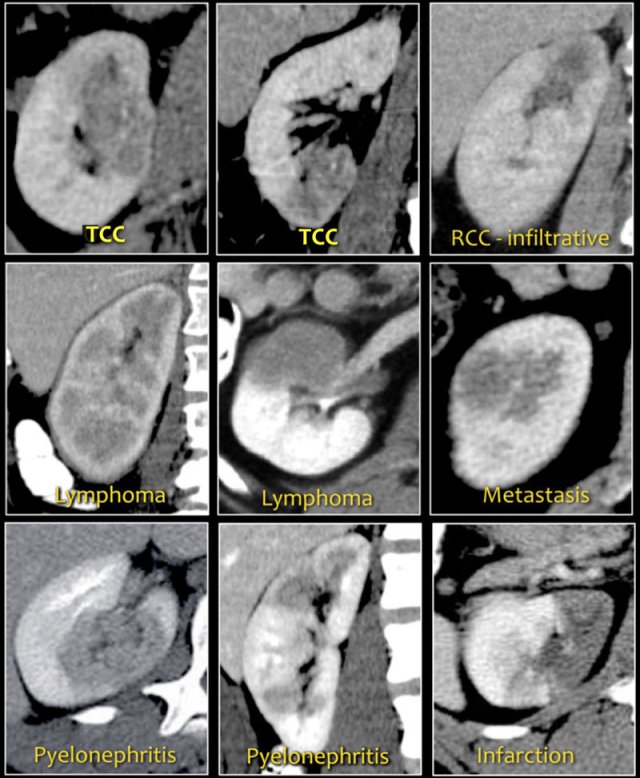
Bean-type lesions
The radiologic features of bean-type lesions are generally nonspecific.
Notice the similar appearance of the lesions in the figure.
The differential diagnosis can often be suggested by integrating clinical and imaging data.
- A central infiltrating lesion that fills the renal pelvis in an older patient is most likely a transitional cell carcinoma (TCC), also called urothelial cell carcinoma (UCC).
- An infiltrative mass in a young patient with sickle cell trait is likely to be renal medullary carcinoma.
- Multifocal and bilateral lesions or diffuse renal infiltration in combination with lymphadenopathy and involvement of other organs is suggestive of lymphoma.
- Multifocal and bilateral renal lesions in known malignancy is suggestive of metastases, although in case of a single infiltrative mass, infiltrative renal cell carcinoma must be considered.
- In patients with pain and signs of infection, the diagnosis is pyelonephritis.
- Wedge-shaped lesions are suggestive of infarction.
Size of a tumor
The size of a tumor is regarded as the most important predictor of malignancy and aggressive histologic grade (1).
The risk of metastatic disease depends on the size of the tumor.
If the size of a tumor is less than 3 cm the risk of metastatic disease is negligible.
Most incidentally found renal masses are small, defined as < 4 cm.
Many of these masses will be either low grade RCC, indolent malignancies or benign lesions.
In renal masses of 1-2 cm which were surgically removed, 56% had a benign histology. Only 13% of masses 6-7 cm had a benign histology.
The growth rate of a small renal mass on serial imaging however has not been shown to provide reliable prediction of malignancy or benignity.
Renal cell carcinoma
Renal cell carcinoma (RCC) is a typical ball-type lesion.
50% of RCCs are incidental findings on imaging studies performed for non-urinary tract symptoms.
Peak incidence of RCC is between 60 and 70 years.
RCC is associated with hereditary syndromes, such as von Hippel-Lindau, tuberous sclerosis and Birt-Hogg-Dubé.
The most common subtype of RCC is clear cell carcinoma, followed by papillary and chromophobe RCC.
Multilocular cystic RCC is uncommon and discussed here.
Renal medullary carcinoma is also very uncommon and occurs almost exclusively in patients with sickle cell trait.
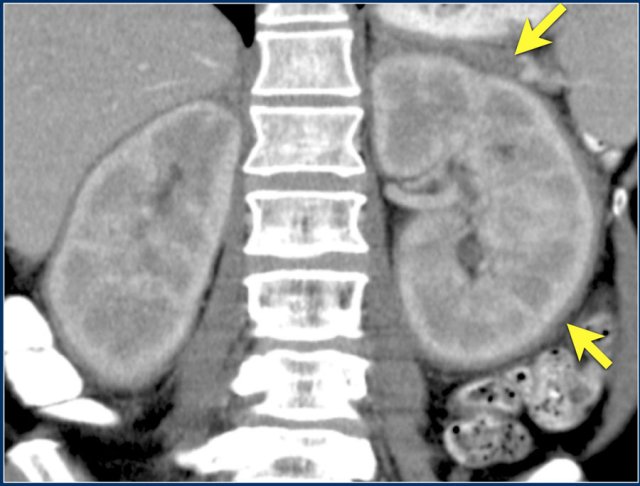
Clearcell RCC
This is the most common subtype of RCC, accounting for 70% of all RCCs.
These tumors arise from the renal cortex and are often expansive.
It is a hypervascular lesion, frequently heterogeneous due to necrosis, hemorrhage, cystic components or calcifications.
In rare cases, RCCs can also contain extracellular fat.
In a mass with both calcifications and fat, consider the possibility of a RCC.
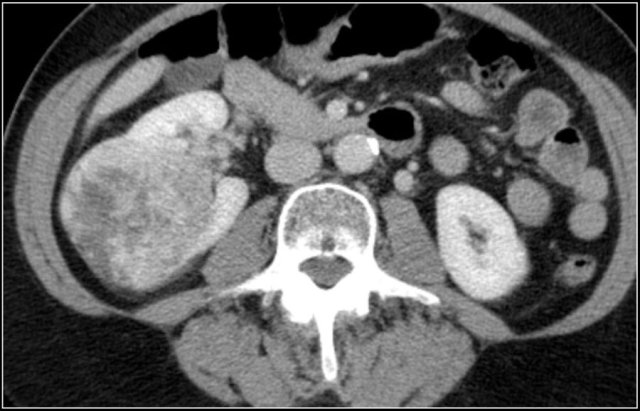
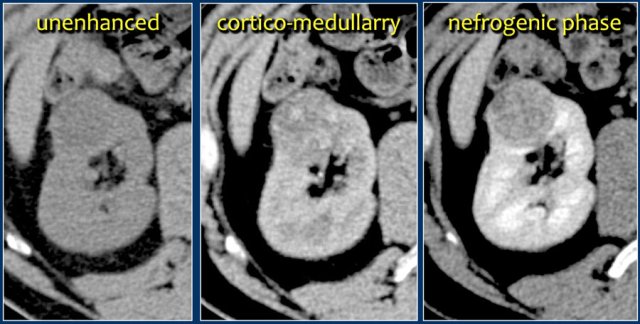
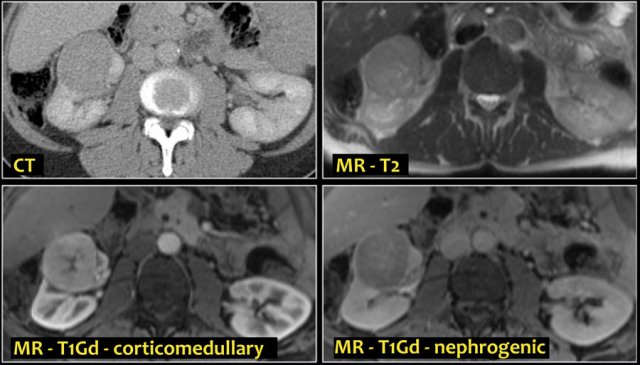
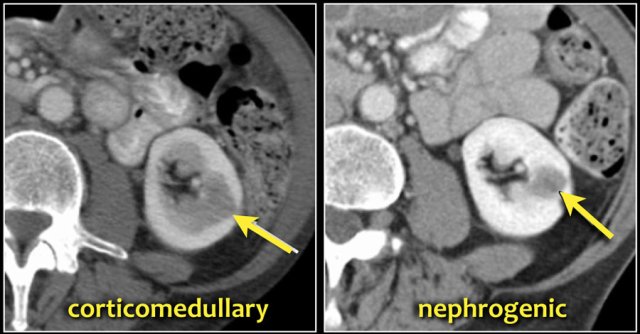
A typical feature of clear cell carcinoma is strong enhancement in the corticomedullary phase.
This can be difficult to assess when the lesion is small and located in the renal cortex, which also enhances strongly.
The nephrogenic phase is therefore the most sensitive phase for the detection of these lesions, as the renal parenchyma enhances homogeneously and more intensely than the tumor (figure).
On MR clear cell RCC is usually iso- to hypointense on T1 and hyperintense on T2-weighted images.
Typically renal cell carcinomas do not have extracellular fat, which differentiates them from angiomyolipomas.
However 80% of clear cell RCC have intracellular fat, which leads to a drop in signal intensity on T1 opposed-phase images compared to in-phase images.
Do not confuse this finding for extracellular fat and do not make the mistake to conclude that you are dealing with an angiomyolipoma.
Von Hippel-Lindau disease is associated with development of clear cell RCCs, often bilateral or multifocal.
Patients with a clear cell RCC have a 5-year survival of 50-60%, which is worse than papillary or chromophobe RCC.
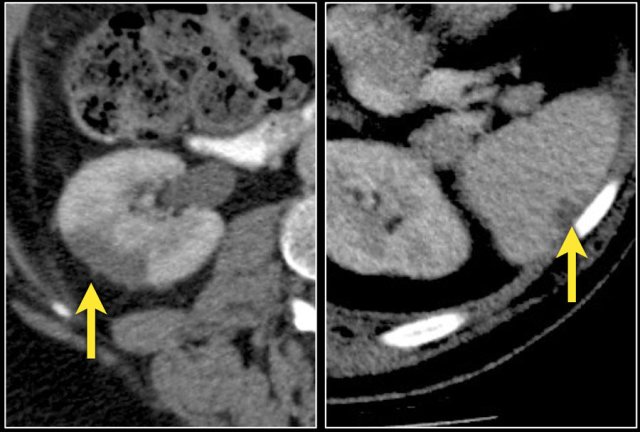
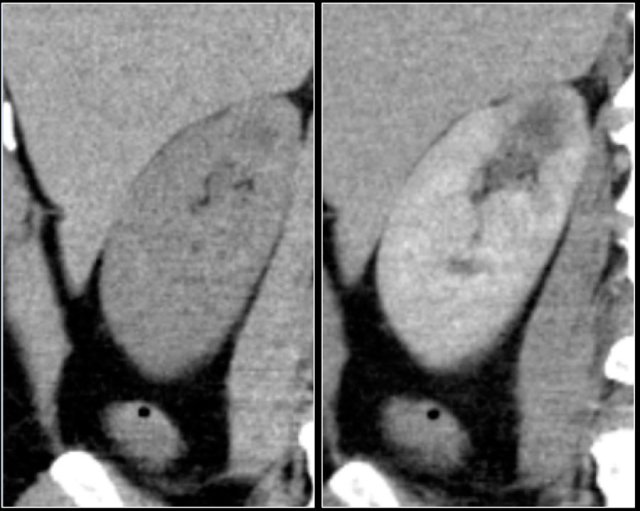 Infiltrative lesion in the upper pole of the right kidney, hardly visible on the unenhanced image on the left, clearly visible in the nephrogenic phase shown on the right. PA showed clearcell RCC
Infiltrative lesion in the upper pole of the right kidney, hardly visible on the unenhanced image on the left, clearly visible in the nephrogenic phase shown on the right. PA showed clearcell RCC
Of all the clear cell RCCs, around 5% have an infiltrative growth pattern.
While this is only a small portion of the RCCs, the overall prevalence of RCC makes it an important consideration for the differential diagnosis of an infiltrative bean-type lesion.
Infiltrative RCCs are aggressive and hypervascular.
It alters the internal architecture of the kidney, preserving the renal contour. Collecting system abnormalities can be similar to those seen in transitional cell carcinomas.
Papillary RCC
Papillary RCC accounts for 10-15% of all RCCs.
These lesions are typically homogeneous and hypovascular and can therefore mimic cysts.
In contrast to clear cell carcinoma, the enhancement of papillary renal cell carcinoma can be very subtle, up to only 10-20 HU difference between unenhanced and enhanced images.
Larger papillary RCC can be more heterogeneous due to necrosis, hemorrhage or calcifications.
On MR they are frequently iso- to hypointense on T1 and hypointense on T2-weighted imaged.
In very rare cases macroscopic fat is encountered in the lesion, often with calcifications.
Bilateral and multifocal tumors are more frequently seen in papillary RCC than in other types of RCC.
The 5-year survival is 80-90%.
Chromophobe RCC
5% of the RCCs are of the chromophobe type.
It is a solid, sharply demarcated and sometimes slightly lobulated lesion.
They can have a central scar or spoke-wheel pattern of contrast enhancement, similar to oncocytomas.
It is not possible to differentiate chromophobe RCC from an oncocytoma on imaging.
Even the histology of these tumors share similar characteristics.
The enhancement of a chromophobe RCC is often homogeneous and less intense than in clear cell RCC.
The prognosis of a chromophobe RCC is similar to the prognosis of papillary RCC with a 5-year survival of 80-90%.
Chromophobe RCC can be seen in patients with Birt-Hogg-Dubé syndrome.
Birt-Hogg-Dubé syndrome is a rare disorder.
These patients have small papular skin lesions called fibrofolliculomas, lung cysts with spontaneous pneumothorax and a high incidence of different kinds of renal cell carcinoma: most frequently chromphobe RCC, less commonly oncocytoma, and rarely clear cell carcinoma.
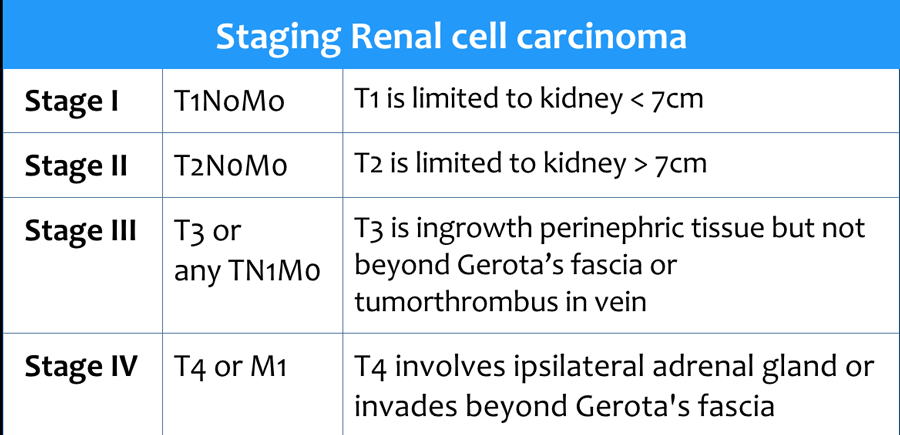
Staging RCC
RCC can invade the perinephric fat beyond the renal fascia and can extend into the renal vein, inferior vena cava or the adjacent adrenal gland.
For the surgeon it is important to know whether there is tumor thrombus in the IVC and if it extends into the chest above the diaphragm (need for a thoracic surgeon during the operation).
Approximately 25% of the patients have metastases at presentation.
Click to enlarge and scroll through images of a T4 renal cell carcinoma
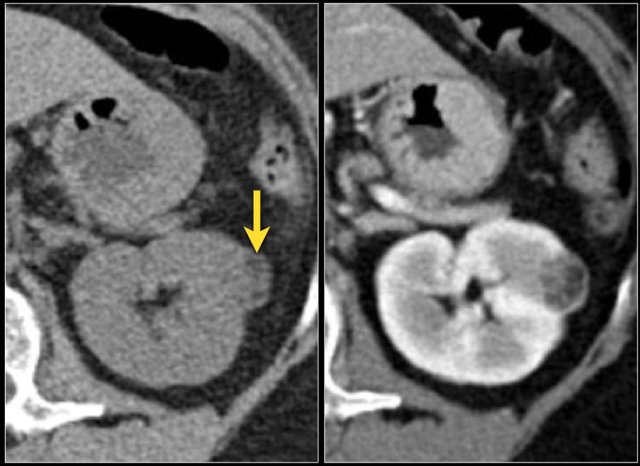
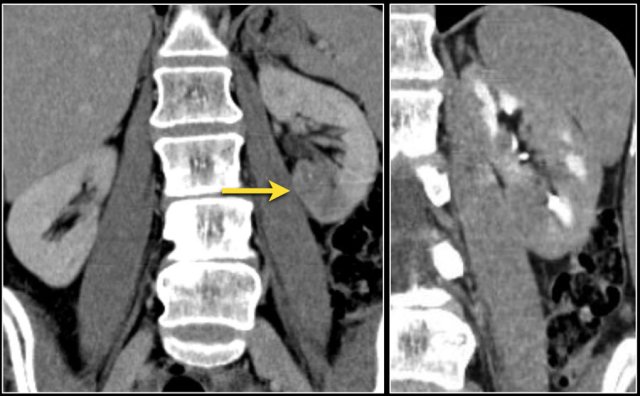
Venous tumor thrombus
The coronal MR-image shows a tumor thrombus extending into the IVC above the diaphragm (arrows).
A thoracic surgeon will be needed during surgery.
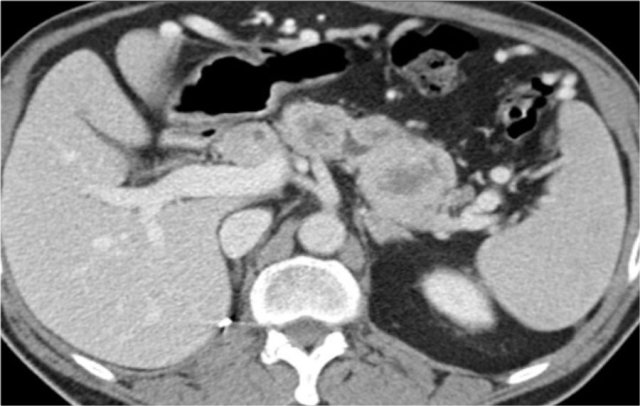
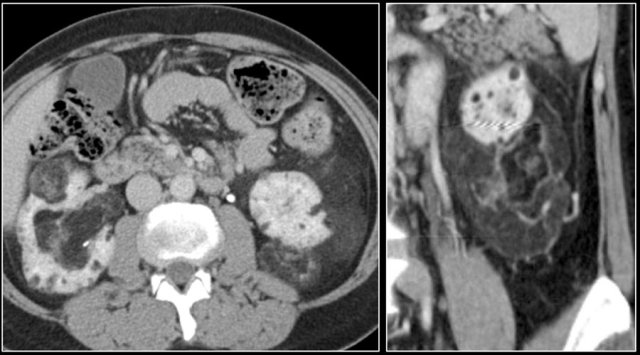
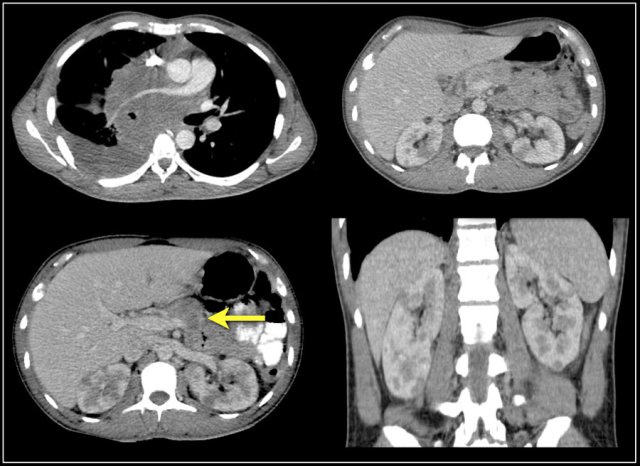
Metastases
25% of the patients with renal cell carcinoma have metastases at presentation.
Common sites are the lung, liver, lymph nodes and bones.
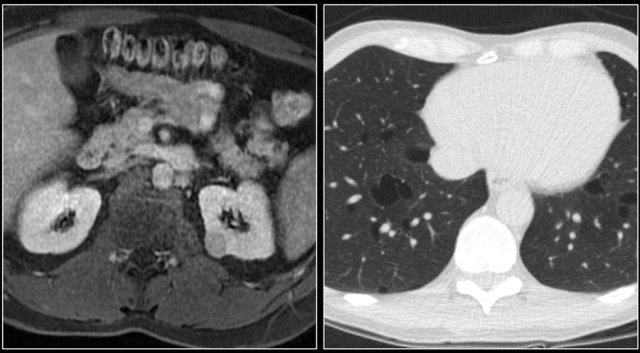
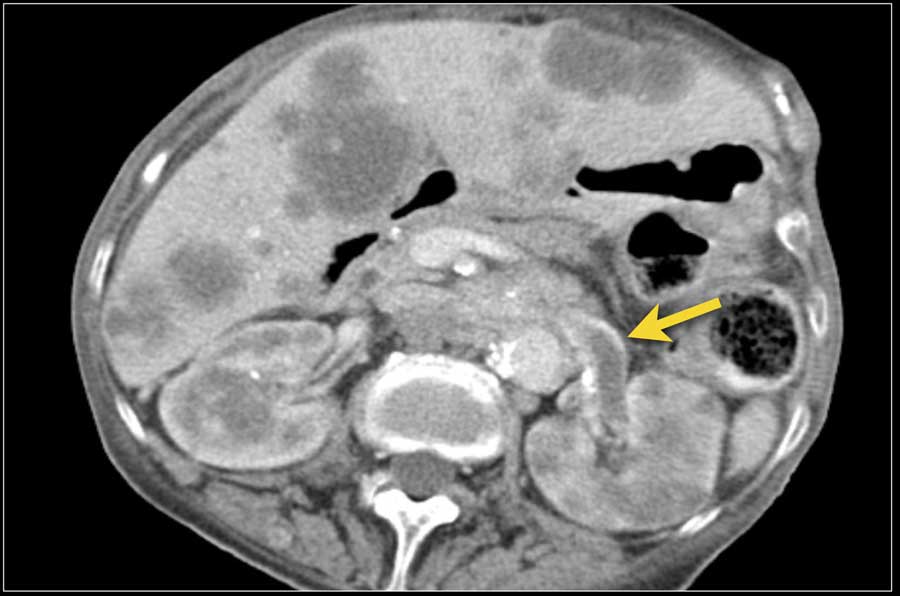
Less common sites are pancreas, adrenal gland, contralateral kidney, mesentery, abdominal wall and brain.
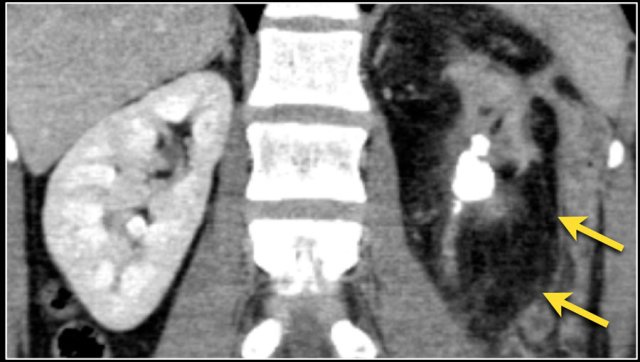
The image shows a patient with metastases in the pancreas.
Angiomyolipoma
Angiomyolipoma (AML) is the most common benign solid renal mass.
It is composed of angiomatous tissue, smooth muscle and fat.
If a lesion contains macroscopic fat on CT, the diagnosis of AML can be made.
On CT an AML is usually a well-defined, heterogeneous tumor, located in the renal cortex and containing areas of fat density of -20 HU or less.
Calcification or necrosis within the tumor is rare.
The presence of both fat and calcifications should raise the suspicion of a RCC.
Enhancement is seen in the vascular and smooth muscle portions of the lesion.
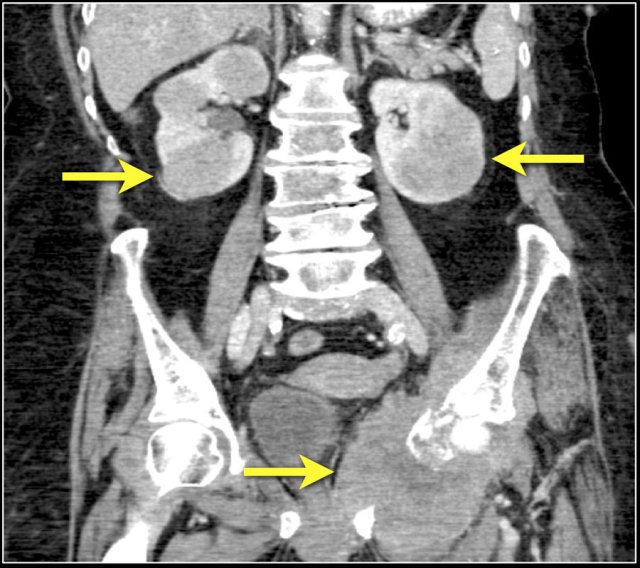
Multiple Angiomyolipomas
Sporadic AML is typically small, unilateral and asymptomatic, usually seen as an incidental finding.
In 10-20% of cases angiomyolipomas are multiple and bilateral.
This is mainly seen in patients with tuberous sclerosis.
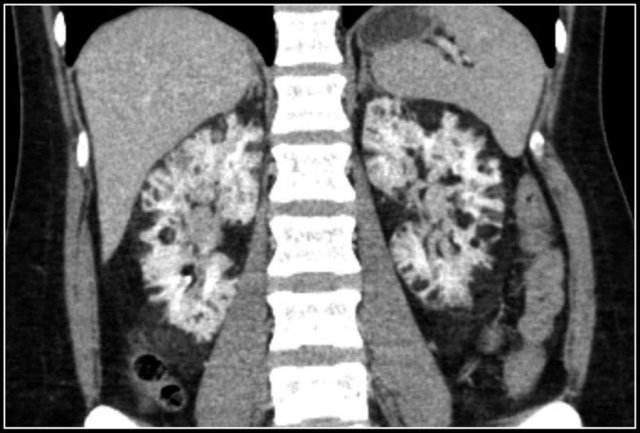
Bleeding in Angiomyopiloma
Due to the abnormal vessels within an AML, it is prone to bleeding.
Patients can present with acute flank pain due to spontaneous hemorrhage.
The risk of hemorrhage increases with size.
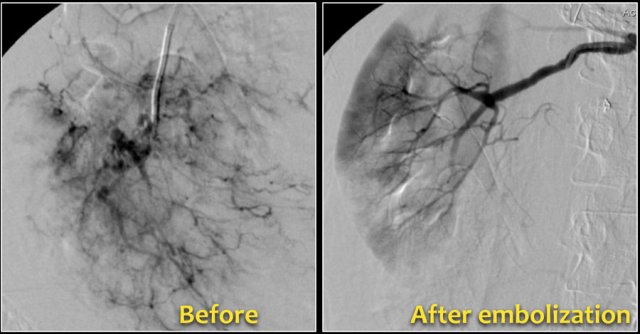
Embolization was performed to stop the bleeding.
Preventive embolization is recommended in tumors larger than 4 cm, even in asymptomatic patients.
Notice the large vessels in the AML in the left kidney.
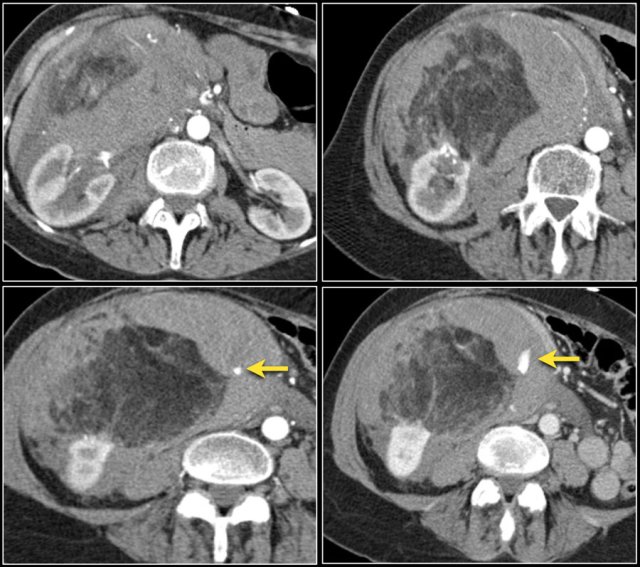
Minimal fat Angiomyolipoma
In 5% of AMLs there is no detectable fat on CT.
The fat can be obscured by internal hemorrhage or it can be a so called minimal fat AML.
On MR macroscopic fat in an AML gives low signal on fat-suppressed images.
Microscopic or intracellular fat, seen as a drop in signal intensity on T1 opposed-phase images compared to in-phase images, is not specific for AML, but can also be present in renal cell carcinoma.
Since the fat is likely to be intracellular in RCC, it is unlikely to be visible on CT images.
Oncocytoma
Oncocytoma is the second most common benign solid renal mass.
3-7% of all solid renal tumors are oncocytomas.
They appear as sharply demarcated lesions with uniform enhancement at CT and often have a central scar.
The central scar can not be distinguished from a central scar or central necrosis in a RCC, therefore oncocytoma is the most commonly excised benign solid mass.
Calcifications in an oncocytoma are rare.
The tumor is usually solitary, 2-12 cm in diameter, but can be multifocal and bilateral.
In less than 10% of cases oncocytoma and chromophobe RCC occur simultaneously.
Transitional cell carcinoma
Transitional cell carcinoma (TCC) also known as urothelial cell carcinoma (UCC) arises from the epithelial cells lining the urinary tract.
Most frequently the TCC arises in the renal pelvis, as a low-grade, superficial tumor, producing a focal intraluminal mass in the renal collecting system.
Approximately 15% of the TCCs are of a more aggressive type with infiltrative growth, altering the regional architecture of the adjacent renal sinus and renal parenchyma, without changing the renal contour.
TCC is a typical bean-type lesion (see figure).
Upper-tract TCC has a peak incidence in the 60- to 70-year age group and is twice as common in men as in women.
Risk factors are smoking, chemical carcinogens, cyclophosphamide therapy and analgesic abuse, particularly long-term use of phenacetin.
TCC is hard to detect on unenhanced CT images.
The nephrogenic phase is the optimal phase to show the interface between TCC and normal enhancing renal parenchyma.
Excretory phase images show collecting system abnormalities such as dilated calyces, calyces distended by tumor or unopacified calyces due to tumor infiltration.
TCC can be locally aggressive with invasion of the retroperitoneum. Regional lymphadenopathy and distant metastases to the lungs and bones may be encountered.
TCC is commonly multifocal with a high incidence of recurrence, therefore requiring thorough surveillance.
TCC has a greater risk of seeding after percutaneous biopsy than RCC, therefore it is not recommended to perform biopsy when there is suspicion of TCC.
Lymphoma
The kidney is a common extranodal site of lymphoma involvement, especially in Non-Hodgkin lymphoma.
Primary involvement of the kidney is rare.
Renal lymphoma usually presents as multiple poorly enhancing masses, but may also present as retroperitoneal tumors directly invading the kidneys or as perirenal soft-tissue masses.
Diffuse infiltration of the renal interstitium results in nephromegaly and is more common in Burkitt lymphoma.
The image shows bilateral involvement of the kidney and a bone lesion in a patient with B-cell lymphoma.
Here another patient with lymphoma located in the mediastinum, pancreas (arrow) and in both kidneys.
Diffuse enlargement of both kidneys in a patient with lymphoma.
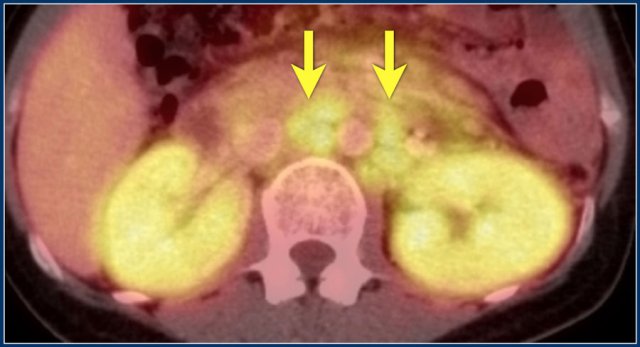
Continue with the PET-CT.
PET-CT shows diffuse renal involvement and also positive periaortic lymph nodes (arrows).
Metastases
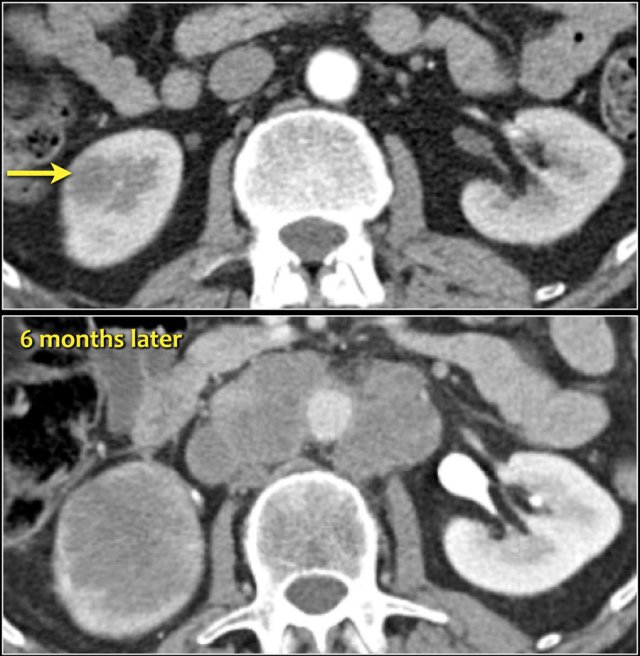 Infiltrative lesion in the lower pole of the right kidney, which has considerably grown six months later, with extensive lymphadenopathy. This turned out to be a metastasis of a lungcarcinoma.
Infiltrative lesion in the lower pole of the right kidney, which has considerably grown six months later, with extensive lymphadenopathy. This turned out to be a metastasis of a lungcarcinoma.
Primary malignancies that most commonly metastasize to the kidney are lung, breast, gastrointestinal tumors and melanoma.
Renal metastases usually occur late in the course of a known malignancy as part of widespread disease.
In rare cases a renal metastasis may manifest as a solitary lesion and may be hard to differentiate from a renal cell carcinoma.
A percutaneous biopsy can be performed to solve this problem.
Renal metastases are typically small, multifocal and bilateral, with an infiltrative growth pattern.
They show mild enhancement, much less than that of the normal renal parenchyma.
Metastases can also be hypervascular however, as in melanoma, and sometimes breast cancer.
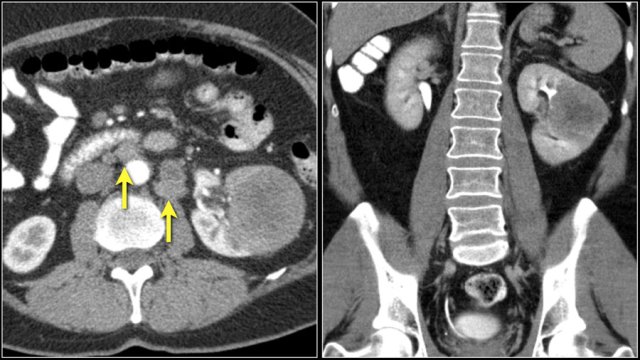
The image shows a patient with multiple renal metastases.
Notice the tumor thrombus in the left renal vein.
Here a patient with lung cancer.
There is a metastasis in the left kidney and there are multiple lymph node metastases (arrows).
If this was the only presentation, it would be difficult to differentiate from a renal cell carcinoma with lymph node metastases.
Infection
Pyelonephritis and renal abscess can be tumor mimics, but in most cases the history and the clinical findings help you to make the right diagnosis.
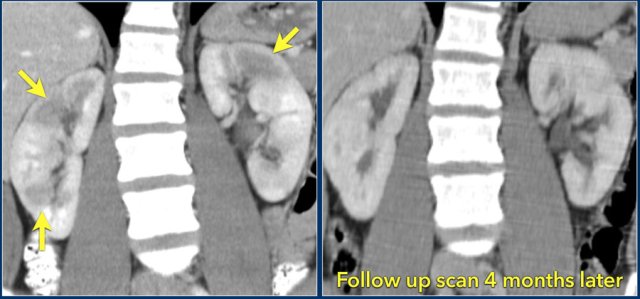
In this case there are hypodense areas in both kidneys.
Based on the imaging alone the main differential is multifocal pyelonephritis, lymphoma and metastases.
This patient had a urinary tract infection and episodes of flank pain and there was no history of a primary tumor or lymphoma.
So the diagnosis is pyelonephritis.
A CT scan 4 months later shows normal enhancement of both kidneys; the renal abnormalities on the first scan were therefore consistent with an episode of multifocal pyelonephritis.
Renal abscess is usually a complication of acute pyelonephritis and patients present with urinary tract infection, flank pain and fever.
On CT a renal abscess usually presents as a non-specific homogeneous low attenuation lesion or as a complex cystic lesion.
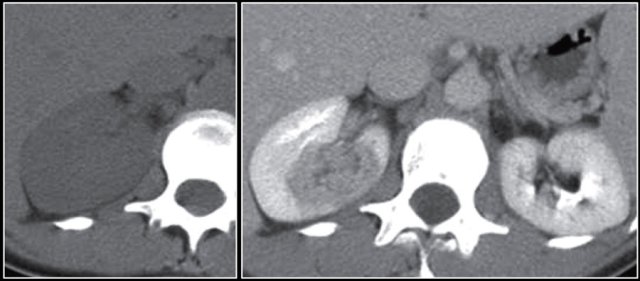
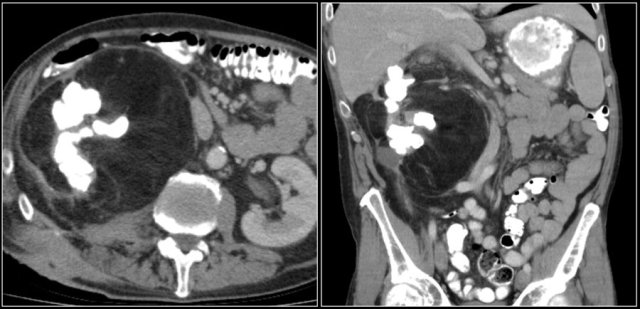
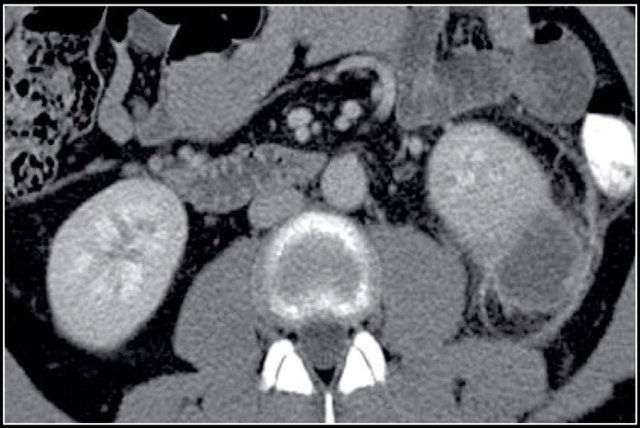 Cystic lesion with thick enhancing rim and infiltration of perirenal fat in a patient with a renal abscess
Cystic lesion with thick enhancing rim and infiltration of perirenal fat in a patient with a renal abscess
The renal abscess may have a thick irregular enhancing wall with infiltration of the perirenal fat (figure).
In patients with an atypical clinical presentation the complex cystic appearance and extension into the perirenal fat may simulate a renal cell carcinoma.
This patient had a typical presentation with right flank pain and laboratory findings consistent with a urinary tract infection.
The ultrasound image shows a hypoechogenic lesion with some echolucency, which indicates a fluid-containing component.
This proved to be an abscess.
Xanthogranulomatous pyelonephritis
Xanthogranulomatous pyelonephritis an uncommon condition caused by a chronic granulomatous infection with accumulation of lipid-laden macrophages.
Frequently there is an obstructing stone with dilatation of calyces.
It results in diffuse renal destruction, but can be segmental as well.
Renal enlargement is present in all patients and in some cases macroscopic fat can be seen.
Here another example of a xanthogranulomatous pyelonephritis.
There is destruction of the right kidney, multiple calculi and surrounding fibrofatty proliferation.
This appearance may resemble a liposarcoma.
Infarction
Renal infarction usually results from thromboembolismin cardiovascular disease.
The common clinical presentation is acute flank pain and hematuria.
In the acute phase CT will show a wedge-shaped area of decreased attenuation followed in a later stage by atrophy.
When the whole kidney is infarcted, the kidney will be enlarged and of low attenuation.
Only the outer cortex may still enhance through collaterals resulting in a cortical rim sign.
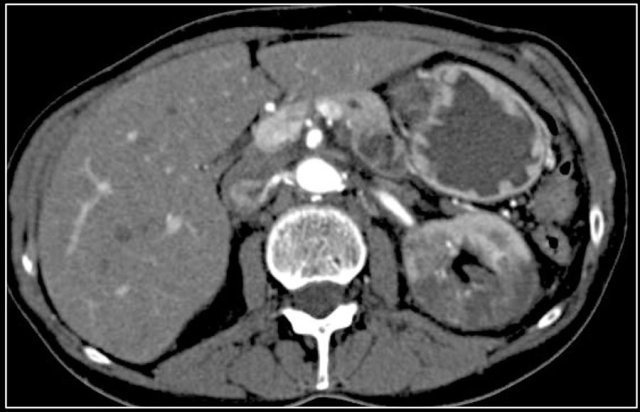
Here another case of renal infarction.
Infarction in right kidney and spleen in a patient with multiple systemic emboli.
Pitfalls
Pseudo-enhancement
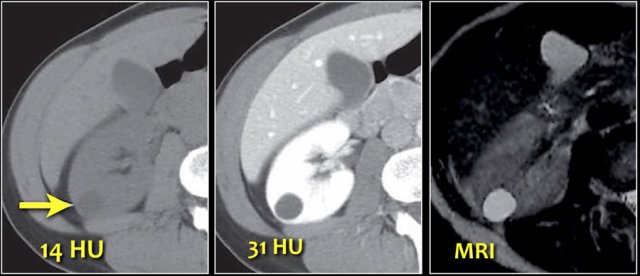
Pseudo-enhancement is a pitfall to be aware of in the evaluation of renal masses.
As mentioned before a small difference in Hounsfield units (< 20 HU) can be measured in a renal cyst on contrast-enhanced CT images due to beam-hardening.
This case shows pseudo-enhancement in a lesion which proved to be a cyst at MRI.
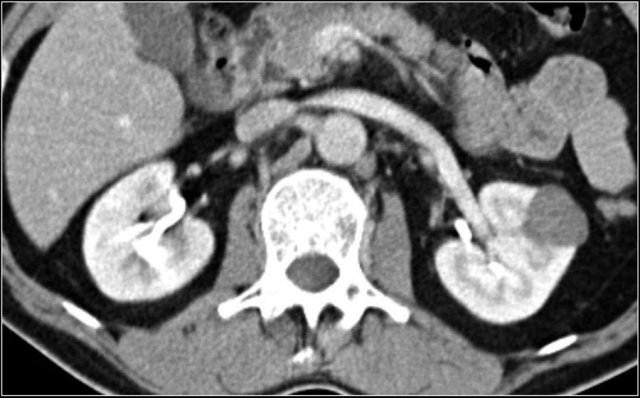
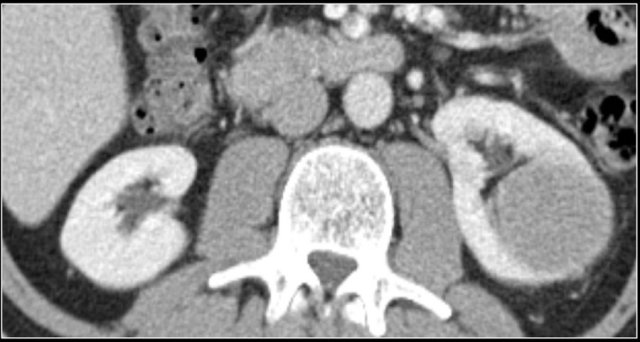
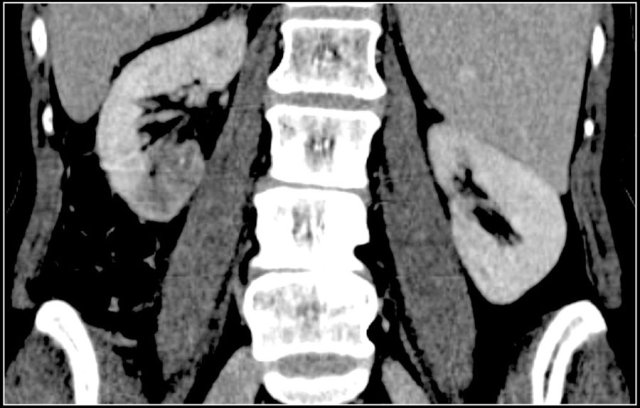
Dromedary hump
Prominent columns of Bertin, bulging of the renal contour and focal renal hypertrophy can look like a renal mass on ultrasound, unenhanced images and CT in the nephrogenic phase.
In the corticomedullary phase the normal corticomedullary pattern in these pseudotumors can be appreciated, distinguishing them from real lesions.
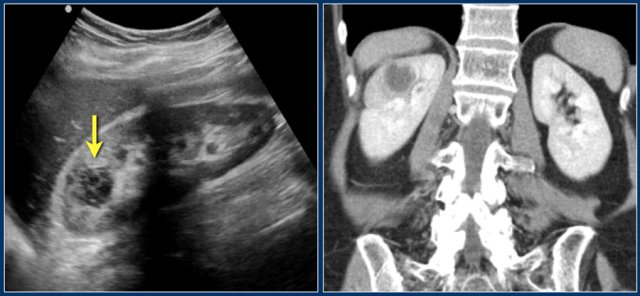
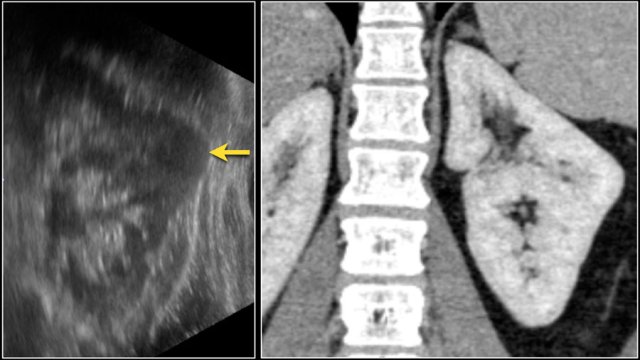
Suspected renal mass of the left kidney on ultrasound.
CT shows a bulging of the left renal contour, commonly referred to as a dromedary hump.
Here is another case.
In the nephrogenic phase one could argue there is a lesion in the left kidney.
In the corticomedullary phase however it is clear that this is a pseudotumor.
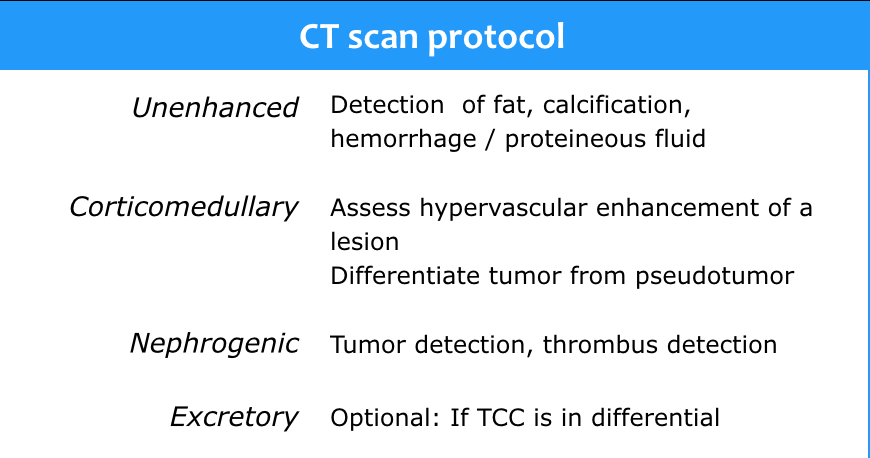
CT protocol
CT is the first choice for characterization of a renal mass and for staging.
The scanprotocol should at least consist of unenhanced images and a scan in the nephrogenic phase.
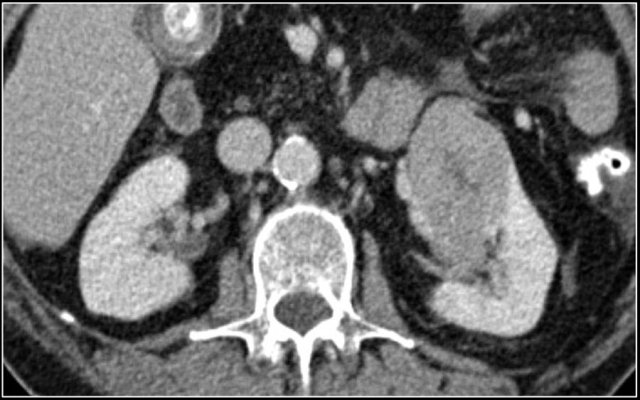
The corticomedullary phase 25-40 sec post injection is strongly recommended. It helps to differentiate tumor from pseudotumor and to assess enhancement of a lesion.
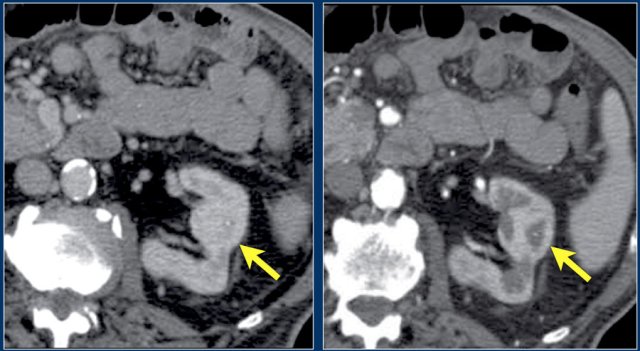
In this phase however a tumor located in the renal medulla can have the same attenuation as the surrounding parenchyma (figure).
Therefore the nephrogenic phase (±100 sec post injection) is the most important phase for the detection of a tumor.
It also provides information on possible vascular involvement and tumor thrombus.
The excretory phase (± 8 min p.i.) is optional to visualize the renal collecting system, ureters and bladder.
It is not needed for assessing a renal cortical mass, but it should be done if TCC is in the differential diagnosis.
Charity
All the profits of the Radiology Assistant go to Medical Action Myanmar which is run by Dr. Nini Tun and Dr. Frank Smithuis sr, who is a professor at Oxford university and happens to be the brother of Robin Smithuis.
Click here to watch the video of Medical Action Myanmar and if you like the Radiology Assistant, please support Medical Action Myanmar with a small gift.