Ischemic and non-ischemic cardiomyopathy
Wouter van Es, Hans van Heesewijk, Benno Rensing, Jan van der Heijden and Robin Smithuis
Radiology and Cardiology department of the St. Antonius Hospital in Nieuwegein and the Rijnland hospital in Leiderdorp, the Netherlands
Publicationdate
In this presentation we will discuss the MRI features of ischemic cardiomyopathy and non-ischemic cardiomyopathies and the role of late enhancement imaging in differentiating between the various types of cardiomyopathy.
Images can be enlarged by clicking on them.
If a video doesn't work, just click the stop button and then the play button once more.
For proper printing you may have to adjust the print settings of your internet browser.
Introduction
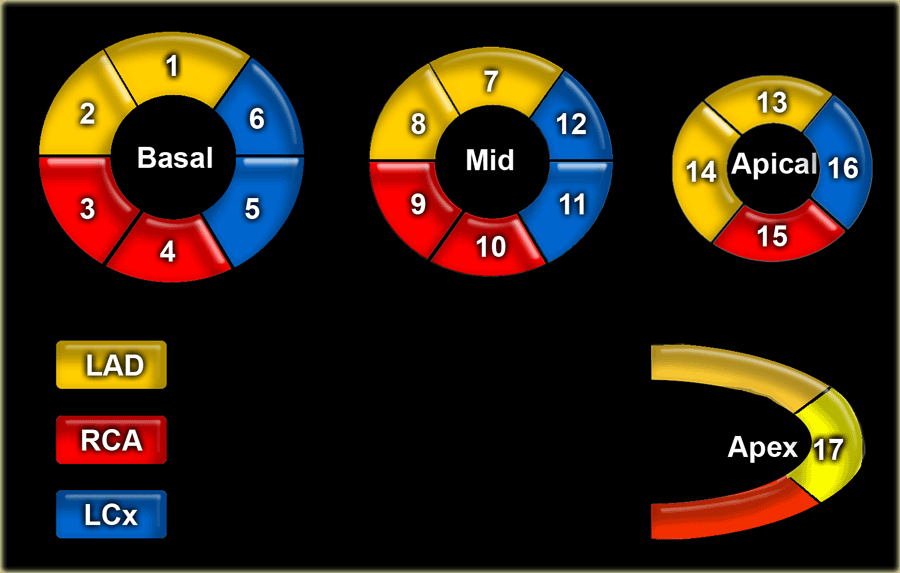
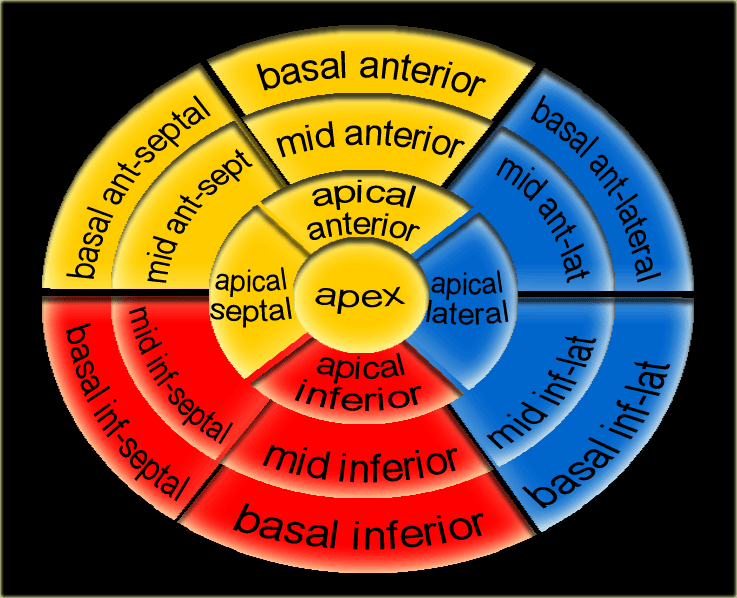
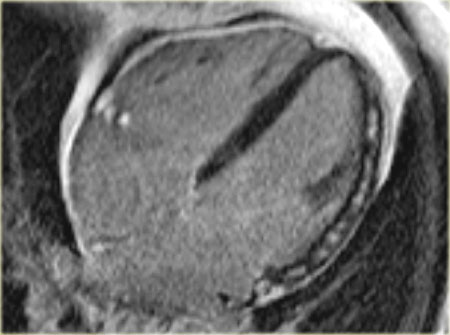
17 segments model
Myocardial segments with abnormal enhancement or wall motion disturbances are named and localized according to the 17 segments model of the American Heart Association (37).
Individual myocardial segments can be assigned to the 3 major coronary arteries with the recognition that there is anatomic variability.
17 segments model (2)
- basal anterior
- basal anteroseptal
- basal inferoseptal
- basal inferior
- basal inferolateral
- basal anterolateral
Enhancement patterns
Administration of Gadolinium results in uptake of the contrast agent into both normal and injured myocardium.
In normal myocardium there will be early wash out of contrast.
In injured myocardium the wash out is very slow resulting in delayed enhancement after 10 - 15 minutes compared to the normal myocardium.
Delayed enhancement of myocardial tissue is seen in many pathophysiologic scenarios:
- Retention of contrast material by fibrous tissue
- Increased extravascular space
- Inflammation
- Tumor neovasculature in primary and secondary tumors
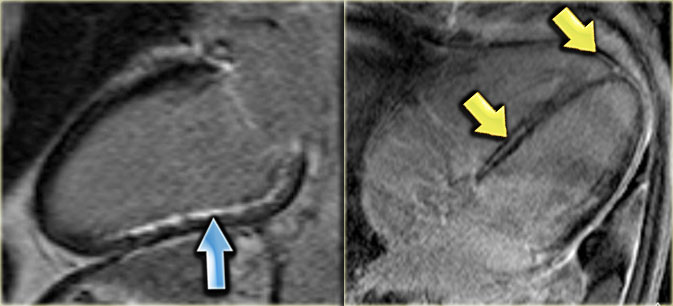 LEFT: Long axis late enhancement image in a patient with an inferior wall infarction with subendocardial enhancement in the territory of the right coronary artery RIGHT: 4-chamber late enhancement image in a patient with idiopathic dilated cardiomyopathy with midmyocardial enhancement
LEFT: Long axis late enhancement image in a patient with an inferior wall infarction with subendocardial enhancement in the territory of the right coronary artery RIGHT: 4-chamber late enhancement image in a patient with idiopathic dilated cardiomyopathy with midmyocardial enhancement
Ischemic versus non-ischemic
The causes of cardiomyopathy (CM) can be divided into ischemic and non-ischemic (1-5).
Ischemic CM
is defined as dysfunction of the left ventricle as a result of a chronic lack of oxygen due to coronary artery disease.
Delayed enhancement MR images will show fibrosis, which appears as high signal intensity in an area of coronary artery distribution.
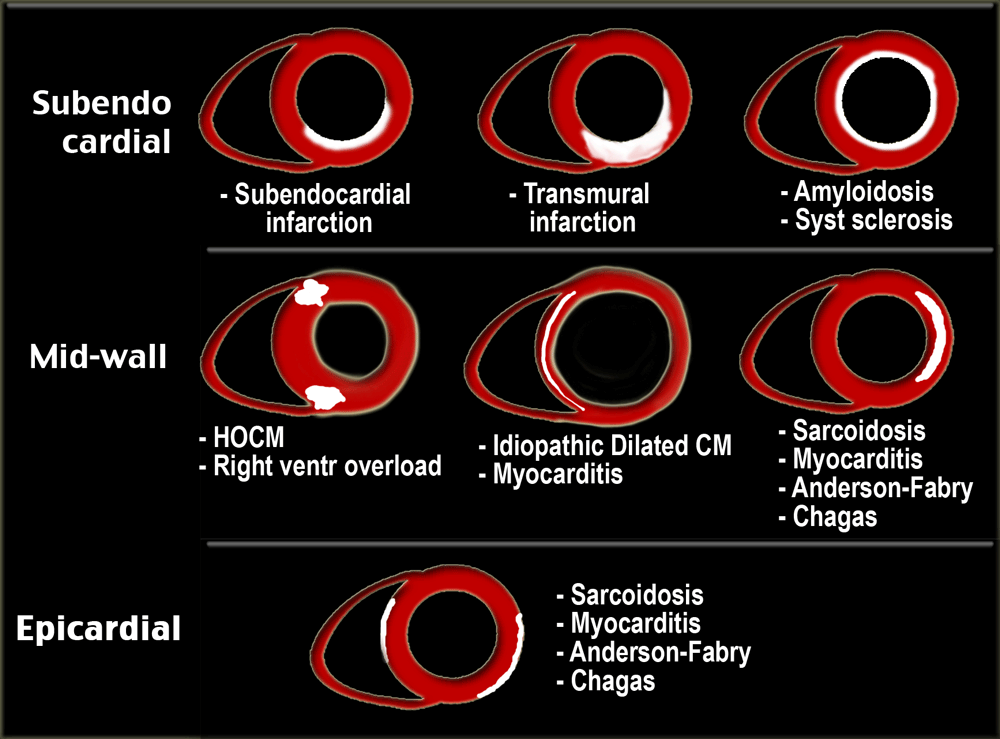
Since all infarctions start subendocardially and may progress to transmural, the subendocardial region is always involved.
Non-ischemic CM
has a variable etiology, i.e. genetic, toxic, metabolic, infectious and idiopathic.
In nonischemic myocardial disease the delayed enhancement usually does not occur in a coronary artery distribution and is often midwall or epicardial rather than subendocardial or transmural.
Ischemic Cardiomyopathy
Infarction and delayed enhancement
Infarcted myocardium is bright on late-enhancement images.
All patients with ischemic cardiomyopathy demonstrate delayed enhancement in a typical 'CAD' pattern, one in which the subendocardium is always involved.
When a coronary artery is occluded the infarction always starts subendocardially and progresses towards the epicardium depending on the duration of the occlusion [6].
Both acute and chronic infarctions enhance.
In acute infarctions the contrast enters the damaged myocardial cells due to myocyte membrane disruption.
In chronic infarctions the late enhancement is a result of retention of contrast material in the large interstitial space between the collagen fibers in the fibrotic tissue [7].
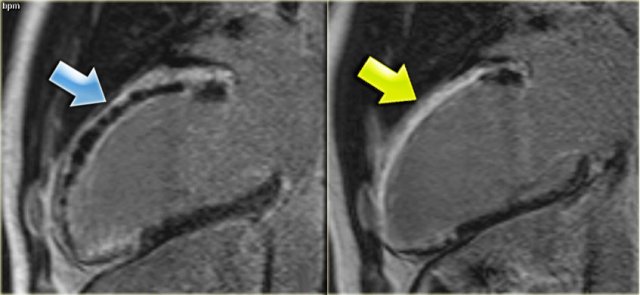 Left: no-reflow phenomenonRight: four months later there is transmural enhancement indicating a transmural infarction
Left: no-reflow phenomenonRight: four months later there is transmural enhancement indicating a transmural infarction
No reflow phenomenon
No reflow phenomenon is the failure of blood to reperfuse an ischemic area after the physical obstruction has been removed or bypassed.
No reflow zones are identified on late-enhancement images as a dark core surrounded by an enhancing rim.
This finding indicates the presence of damaged microvasculature in the core of an area of infarction
The presence of a 'no reflow' zone is associated with worse functional outcome, larger infarcts and adverse clinical outcome [8,9].
Both acute and chronic infarctions demonstrate delayed-enhancement, but an acute infarction can often be distinguished by the presence of a 'no reflow' zone and high signal on T2 weighted images.
Stunning
Cine imaging in combination with delayed-enhancement MR allows identification of:
- Myocardial stunning following acute myocardial infarction and
- Hibernating myocardium in the setting of chronic ischemic heart disease.
Stunning is defined as postischemic myocardial dysfunction that persists despite restoration of normal blood flow.
Over time there can be a gradual return of contractile function depending on the transmurality of the ischemia [10].
If the degree of transmurality as seen on the delayed enhancement images is less than 50%, the myocardial function is likely to recover [11].
On the left a long axis cine 6 days after revascularization of an acute inferior wall infarction.
First study the video and then continue reading.
- There is good contraction of the normal anterior wall
- Despite the revascularization there is hypokinesia of the inferior wall.
Continue with the delayed enhancement image.
On the left the long axis delayed enhancement image of the same patient.
There is less than 50% enhancement of the myocardium.
This is a good prognostic sign and we can expect a restoration of some of the contractile function.
Continue with the cine-view four months later.
On the left the same patient four months after the inferior infarction and revascularization.
First study the video and then continue reading.
The long axis cine shows improved function of the inferior wall.
Now it can be concluded that the hypokinesia was due to stunning.
Myocardial regions that demonstrate little or no evidence of hyperenhancement (i.e. infarction) have a high likelihood of recovery, whereas regions with transmural hyperenhancement have virtually no chance of recovery.
Hibernation
Hibernation is a state in which some segments of the myocardium exhibit abnormalities of contractile function at rest [10].
This phenomenon is highly significant clinically because it usually manifests itself in the setting of chronic ischemia, that is potentially reversible by revascularization.
The reduced coronary blood flow causes the myocytes to enter a low-energy 'sleep mode' to conserve energy.
There is an inverse relationship between the transmural extent of hyperenhancement, and the likelihood of wall motion recovery following revascularization.
If the transmural extent of late enhancement is less than 50% the function is likely to improve after revascularization [12].
On the left long axis cine-images of a patient with a severe stenosis of the LAD.
First study the video and then continue reading.
The cine images show:
- Hypokinesia of the anterior wall.
- Hypokinesia of the inferior wall.
-
Poor ejection fraction: 17%.
Continue with the late enhancement image.
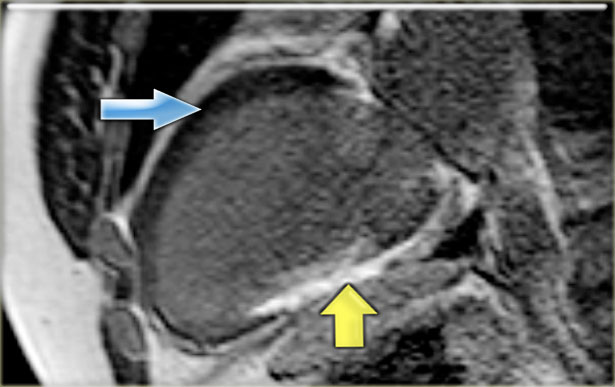 Hibernation of the anterior wall (blue arrow) and old transmural inferior wall infarction (yellow arrow).
Hibernation of the anterior wall (blue arrow) and old transmural inferior wall infarction (yellow arrow).
On the left the long axis late enhancement image in the same patient.
Noice the following:
- Transmural enhancement of the inferior wall, which can be diagnosed as an old infarction (yellow arrow)
-
No enhancement of the hypokinetic anterior wall (blue arrow).
So it can be concluded, that this is probably the result of hibernation.
After PTCA there is improvement of the function of the anterior wall.
The ejection fraction improved from 17 to 49%.
Non Ischemic cardiomyopathy
Non Ischemic cardiomyopathy is defined as a myocardial disorder in which the heart muscle is structurally and functionally abnormal, in the absence of other causes of heart dysfunction, like coronary artery disease, hypertension, valvular disease and congenital heart disease.
We will discuss the cardiomyopathies listed in the table on the left.
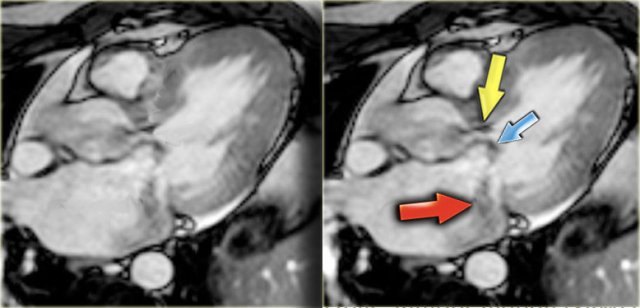 HCM with narrow left ventricular outflow tract (yellow arrow), systolic anterior motion of anterior leaflet of mitral valve (blue arrow) and mitral regurgitation (red arrow)
HCM with narrow left ventricular outflow tract (yellow arrow), systolic anterior motion of anterior leaflet of mitral valve (blue arrow) and mitral regurgitation (red arrow)
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is characterized by a hypertrophied left ventricle, defined as diastolic wall thickness 15mm or more, without any identifiable cause such as hypertension or valvular disease.
Normal ventricular septal measurement is 8-12 mm.
Usually there is asymmetric thickening of the wall most prominently involving the ventricular septum without abnormal enlargement of the ventricular cavities.
It is a genetic myocardial disorder with a prevalence of 1:500.
In about 25% of patients there is obstruction of the left ventricular outflow tract (LVOT) due to hypertrophy of the basal septum and a systolic anterior motion of the mitral valve (SAM).
In these cases the term HOCM or hypertrophic obstructive cardiomyopathy is used.
The systolic anterior motion of the mitral valve is probably the result of the increased flow velocity and decreased pressure above the valve caused by the hypertrophied interventricular septum (the Venturi effect).
In the vast majority of patients the systolic anterior motion of the mitral valva is the mechanism of obstruction in HCM and also the cause of the mitral regurgitation.
On an end-systolic image the following findings can be depicted (figure):
- Jet in the narrowed left ventricular outflow tract
- Systolic anterior motion of the anterior leaflet of the mitral valve
- Mitral regurgitation
HOCM (2)
On the left an end-diastolic image.
The arrow points to the hypertrophic basal septum.
Continue with the 3-chamber view movie.
On the left the 3-chamber view movie of the same patient.
First study the video and then continue reading.
The video nicely demonstrates:
- Systolic anterior motion of the anterior leaflet of the mitral valve
- Mitral regurgitation jet
- Jet in the narrowed left ventricular outlet.
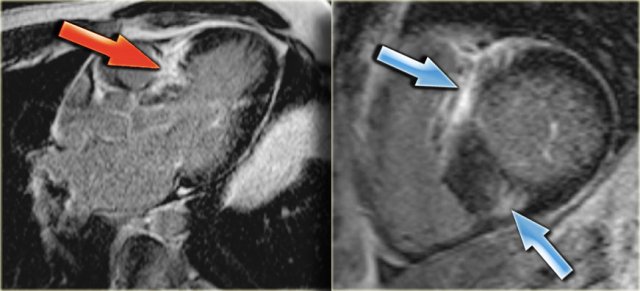 Left: 3-chamber late enhancement image shows the enhancement of the hypertrophic basal septum (arrow)
Left: 3-chamber late enhancement image shows the enhancement of the hypertrophic basal septum (arrow)
HOCM (3)
On the far left a 3-chamber late enhancement image which nicely demonstrates the enhancement of the hypertrophic basal septum (arrow).
Next to it a short axis late enhancement image which demonstrates the typical enhancement at the anterior and posterior right ventricular insertion points (arrows).
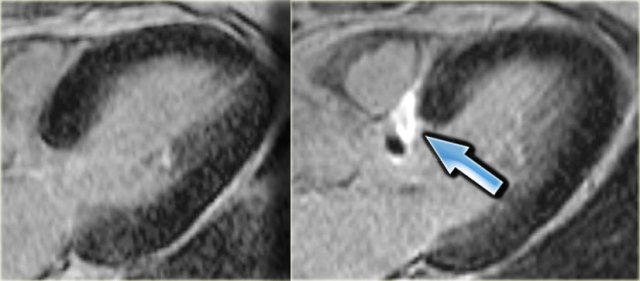 3-chamber late enhancement image before (left) and after (right) alcohol ablation. Note the transmural infarction of the basal septum (arrow).
3-chamber late enhancement image before (left) and after (right) alcohol ablation. Note the transmural infarction of the basal septum (arrow).
The therapy of HOCM is pharmacological, surgical myotomy or alcohol ablation [15].
The results of the alcohol ablation are very well depicted with MRI [19].
On the left a 3-chamber late enhancement image before and after alcohol ablation.
Note the transmural infarction of the basal septum (arrow).
Continue with the 3-chamber movie pre-alcohol ablation.
HOCM (4)
On the left a 3-chamber movie of the same patient before the alcohol ablation.
Notice the systolic anterior movement of the anterior leaflet of the mitral valve and the mitral regurgitation.
On the left the 3-chamber movie post-alcohol ablation with thinning of the basal septum and normalization of the function of the mitral valve.
Restrictive cardiomyopathy - Amyloidosis
The most common cause of restrictive cardiomyopathy is amyloidosis [20].
Amyloid deposits in the myocardium cause abnormal diastolic function with biatrial enlargement, concentric thickening of the left ventricle and reduced systolic function of usually both ventricles.
Cardiac involvement in systemic amyloidosis occurs in up to 50% and has a poor prognosis with a median survival of 6 months [3].
On the left a 4-chamber movie of a patient with amyloidosis.
There is diffuse hypokinesia of the left and right ventricle.
Same patient, short axis movie.
Late enhancement image shows enhancement over the entire subendocardial circumference, variably extending into the neighboring myocardium [21].
Sometimes it is difficult to find the optimal inversion time for nulling the normal myocardium [1].
On the left the 4-chamber and short axis late enhancement images.
There is circumferential subendocardial enhancement extending into the neighboring myocardium.
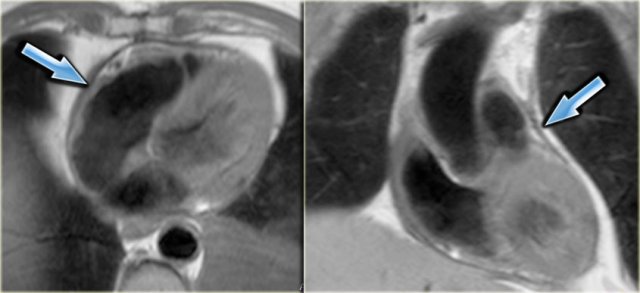 Axial and coronal black-blood images of a patient with constrictive pericarditis after CABG. Arrows point to the thickened pericardium.
Axial and coronal black-blood images of a patient with constrictive pericarditis after CABG. Arrows point to the thickened pericardium.
Constrictive cardiomyopathy
The most important differential diagnosis of restrictive cardiomyopathy is constrictive cardiomyopathy.
MRI can differentiate between those two diagnoses:
- Pericardium is usually thickened in constrictive cardiomyopathy
- Diastolic septal bounce is seen in constrictive, but not in restrictive cardiomyopathy [22,23]
On the left the 4-chamber movie in a patient with constrictive CM.
Notice the diastolic septal bounce which is typical for constrictive cardiomyopathy.
Same patient, short axis movie.
Dilated cardiomyopathy
Dilated cardiomyopathy is defined as dilatation with an end diastolic diameter greater than 55mm measured on the left ventricular outflow image and an ejection fraction < 40%.
Patients with idiopathic dilated cardiomyopathy show either no enhancement or linear midmyocardial enhancement [24].
This enhancement is explained by the presence of fibrosis.
This indicates a poorer prognosis.
Patients with midmyocardial enhancement are at higher risk of sudden cardiac death and arrhythmias [25].
On the left a 4-chamber view of a patient with idiopathic cardiomyopathy.
Notice the mitral regurgitation.
Continue with the late enhancement image.
The late enhancement image does not show any enhancement.
This is compatible with idiopathic dilated cardiomyopathy.
Dilated cardiomyopathy (2)
The differentiation between idiopathic dilated cardiomyopathy and ischemic dilated cardiomyopathy is important, as ischemic cardiomyopathy might be treated with revascularization and idiopathic disease not.
Late enhancement MRI will show subendocardial enhancement in patients with ischemic cardiomyopathy.
On the left a 4-chamber movie of a patient with dilated cardiomyopathy.
Continue with the late enhancement image.
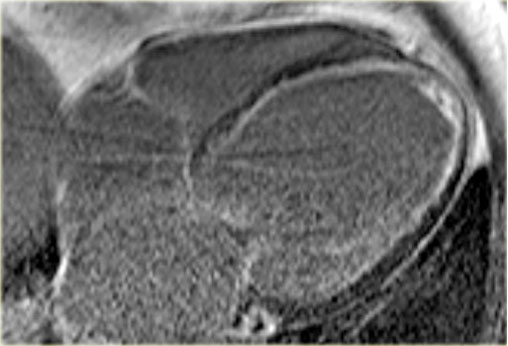 Late enhancement image in a patient with ischemic dilated cardiomyopathy . Note the characteristic subendocardial enhancement.
Late enhancement image in a patient with ischemic dilated cardiomyopathy . Note the characteristic subendocardial enhancement.
The late enhancement MRI shows subendocardial enhancement in this patient.
So we can conclude that this is dilated cardiomyopathy as a result of ischemia.
Dilated cardiomyopathy (3)
In patients with dilated cardiomyopathy it is important to determine the ejection fraction.
According to the guidelines of ACC/AHA/HRS 2008 [26] there is an indication for an automated implantable cardioverter-defibrillator (AICD) if:
- ejection fraction < 35%
On the left the 4-chamber view of a patient with the idiopathic dilated cardiomyopathy.
The ejection fraction was measured to be 28%.
Same patient with the idiopathic dilated cardiomyopathy: short axis view.
Notice the poor contraction.
On the left the late enhancement images of the same patient.
There is midmyocardial septal enhancement consistent with fibrosis.
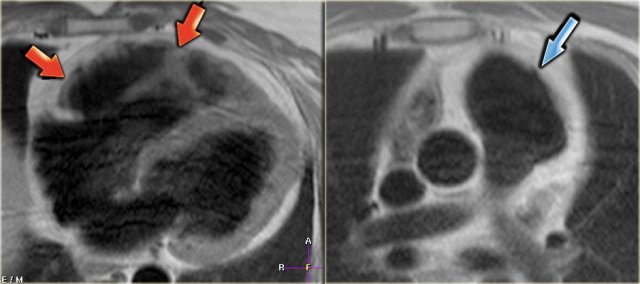 Left: fatty infiltration in the myocardium of the anterior wall of the dilated right ventricle (arrows) Right: dilated right ventricular outflow tract with micro-aneurysm (arrow).
Left: fatty infiltration in the myocardium of the anterior wall of the dilated right ventricle (arrows) Right: dilated right ventricular outflow tract with micro-aneurysm (arrow).
ARVC
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy whose hallmark is fibrofatty replacement of the RV myocardium.
The left ventricle is also involved in at least 15% of patients.
The patients develop progressive RV failure and present with ventricular arrhythmias which can cause sudden cardiac death especially in young people.
Morphologically the right ventricle can have regional wall thinning, hypertrophy, dilatation and microaneurysms.
Functionally cine images are evaluated for RV dysfunction, microaneurysm formation, and focal areas of RV dyskinesia.
MR scans may be overinterpreted since the RV has substantial normal variations including variable trabeculation and small outward bulges near the insertion of the moderator band.
On the left axial black-blood images of a patient with fatty ARVC.
There are two variants of ARVC: fatty and fibro-fatty.
The fatty form is characterized by fatty replacement of the myocardium without thinning of the ventricular wall.
The fibro-fatty form is associated with significant thinning of the right ventricular wall.
The sites of involvement are mostly found in the subtricuspid area, the right ventricular apex, and the infundibulum, the 'triangle of dysplasia' [4].
On the left a 4-chamber movie in a patient with ARVC. Notice the dilated right ventricle with severe segmental hypo- and dyskinesis resulting in small aneurysms.
On the left a short axis movie in a patient with ARVC.
Notice the dilated right ventricle with severe segmental dyskinesis resulting in small aneurysms.
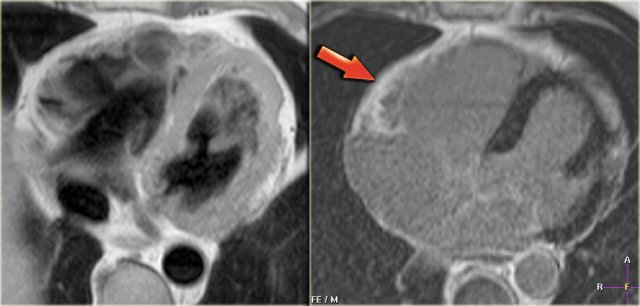 Left: axial black-blood image of a patient with fibro-fatty ARVC Right: late enhancement image shows enhancement of the anterior wall of the right ventricle (arrow).
Left: axial black-blood image of a patient with fibro-fatty ARVC Right: late enhancement image shows enhancement of the anterior wall of the right ventricle (arrow).
ARVC (2)
MRI can show segmental hypokinesis, dilatation, fatty infiltration in the right ventricular myocardium, small aneurysms and late enhancement of the myocardium [5,27].
Fat infiltration is seldom the only abnormality seen on MRI in ARVC, it should coincide with right ventricular regional dysfunction [28].
The diagnosis ARVC cannot be made on MRI findings alone.
On the left a 4-chamber movie of a patient with ARVC.
There is a dilated right ventricle with severe segmental hypokinesis and dyskinesis.
ARVC (3)
The diagnosis is based on major and minor Task Force criteria, many of which involve clinical and laboratory information [29].
Major criteria demonstrated by MRI are:
- localized aneurysms
- severe global or segmental dilatation of the right ventricle
- global systolic dysfunction.
Minor criteria shown by MRI include [27] :
- mild global or segmental dilatation of the right ventricle
- regional contraction abnormalities
- global diastolic dysfunction
Myocarditis
Myocarditis is often caused by a viral infection.
Acute myocarditis can be a cause of sudden cardiac death.
Most patients spontaneously recover, however 5-10% of the patients will develop a dilated cardiomyopathy [30].
Acute myocarditis may clinically mimic an acute myocardial infarction with chest pain.
Abnormal laboratory findings and ECG changes may also suggest an acute coronary syndrome.
The MRI findings however are discriminatory between those two diagnoses. The late enhancement images are key, as the late enhancement in myocarditis is subepicardially or midmyocardially located, and does not originate from the subendocardium [30].
On the left a patient with myocarditis.
Notice the midmyocardial enhancement of the lateral wall.
Same patient with myocarditis.
Notice that the midmyocardial enhancement of the lateral wall has diminished.
Myocarditis (2)
Most lesions with myocarditis occur in the lateral free wall.
Wall motion abnormalities may or may not be present.
There is a potential relationship between the location of late enhancement, the etiologic virus and the prognosis [31].
On the left a patient with myocarditis.
The 4-chamber movie demonstrates hypokinesia of the lateral wall of the left ventricle.
Continue with the movie 10 months later.
4-chamber movie 10 months later.
The lateral wall is now normokinetic.
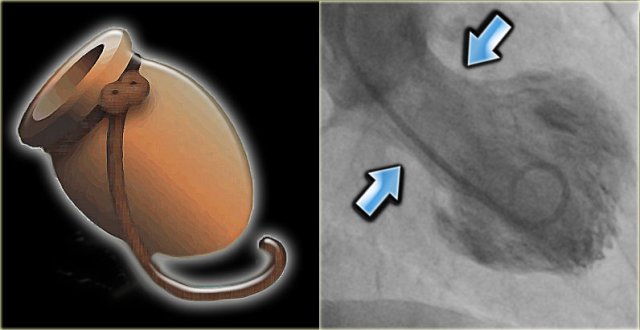 LEFT: Octopus pot RIGHT: left ventricle angiogram in a patient with Tako tsubo cardiomyopathy. There is only contraction of the basal segments (blue arrows) and the mid- and apical segments balloon.
LEFT: Octopus pot RIGHT: left ventricle angiogram in a patient with Tako tsubo cardiomyopathy. There is only contraction of the basal segments (blue arrows) and the mid- and apical segments balloon.
Tako-Tsubo cardiomyopathy
Tako-Tsubo cardiomyopathy or apical ballooning syndrome is a transient cardiomyopathy affecting postmenopausal women after physical or emotional stress.
Patients present with symptoms mimicking an acute myocardial infarction.
The ECG changes and abnormal laboratory findings may also mimic an infarction.
However, coronary angiography is usually normal, but if a left ventricle angiogram is performed, marked hypokinesia of the apical cardiac segments is noted (figure).
The Japanese word takotsubo means octopus pot.
This pot was used to capture octopus and resembles the shape of the left ventricle during systole in these patients
These apical wall motion abnormalities are well seen with MRI.
The motion abnormalities are transient and return to normal within weeks.
On the left a patient with Tako-Tsubo cardiomyopathy.
Notice the hypokinesia of the apex.
The apical wall motion abnormalities were transient and returned to normal within weeks.
Continue with the late enhancement image.
Tako-Tsubo cardiomyopathy (2)
Typically there is no late enhancement, which distinguishes it from an infarction [4].
The pathogenesis is unknown, but it is probably caused by the release of catecholamines.
The modified Mayo Clinic criteria for diagnosis of takotsubo cardiomyopathy:
-
Transient hypokinesis, dyskinesis or akinesis of the left ventricular mid-segments with or without apical involvement
The regional wall motion abnormalities extend beyond a single epicardial vascular distribution
A stressful trigger is often present. - Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture.
- New electrocardiographic abnormalities either ST-segment elevation and/or T-wave inversion or modest elevation in cardiac troponin level.
- Absence of pheochromocytoma or myocarditis.