Systematic Approach to Brain Tumors
Robin Smithuis and Walter Montanera
Radiology Department of the Alrijne hospital, Leiderdorp, the Netherlands and the Division of Neuroradiology of the St. Michael's Hospital, University of Toronto, Canada
Publicationdate
This review is based on a presentation given by Walter Montanera and was adapted for the Radiology Assistant by Robin Smithuis.
In this review a systematic approach for the analysis of a possible brain tumor is described.
Introduction
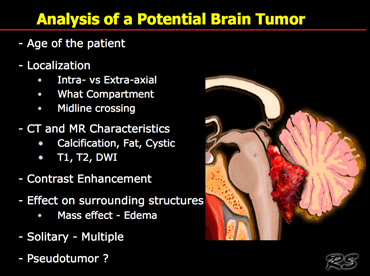
When we analyze a potential brain tumor, there are many questions that need to be answered.
Since different tumors occur in different age groups we first of all need to know the age of the patient.
Next we need to know where the lesion is located - is it intra- or extra-axial and in what anatomical compartment does it lie?
Is it located in the sellar or pontocerebellar region for example?
Is it a solitary mass or is there multi-focal disease?
On CT and MR we look for tissue characteristics like calcifications, fat, cystic components, contrast enhancement and signal intensity on T1WI, T2WI and DWI.
Most brain tumors are of low signal intensity on T1WI and high on T2WI.
Therefore high signal intensity on T1WI or low signal on T2WI can be an important clue to the diagnosis.
Finally we have to consider the possibility that we are dealing with a lesion that simulates a tumor - like an abscess, MS-plaque, vascular malformation, aneurysm or an infarct with luxury perfusion.
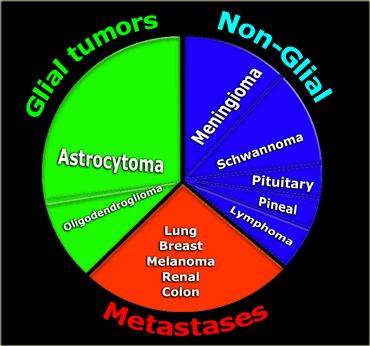
Incidence of CNS tumors
Roughly one-third of CNS tumors are metastatic lesions, one third are gliomas and one-third is of non-glial origin.
Glioma is a non-specific term indicating that the tumor originates from glial cells like astrocytes, oligodendrocytes, ependymal and choroid plexus cells.
Astrocytoma is the most common glioma and can be subdivided into the low-grade pilocytic type, the intermediate anaplastic type and the high grade
malignant glioblastoma multiforme (GBM).
GBM is the most common type (50% of all astrocytomas).
The non-glial cel tumors are a large heterogenous group of tumors of which meningioma is the most common.
Note: since the publication of this article, these numbers have changed and metastases now outnumber the primary brain tumors and are increasing in incidence as overall cancer survival improves.
Age distribution
The age of the patient is an important factor for the differential diagnosis.
Specific tumors occur under the age of 2, like choroid plexus papillomas, anaplastic astrocytomas and teratomas.
In the first decade medulloblastomas, astrocytomas, ependymomas, craniopharyngeomas and gliomas are most common, while metastases are very rare.
When they do occur at this age, metastases of a neuroblastoma are the most frequent.
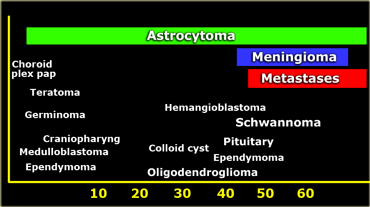
In adults about 50% of all CNS lesions are metastases.
Other common tumors in adults are astrocytomas, glioblastoma multiforme, meningiomas, oligodendrogliomas, pituitary adenomas and schwannomas.
Astrocytomas occur at any age, but glioblastoma multiforme is mostly seen in older people.
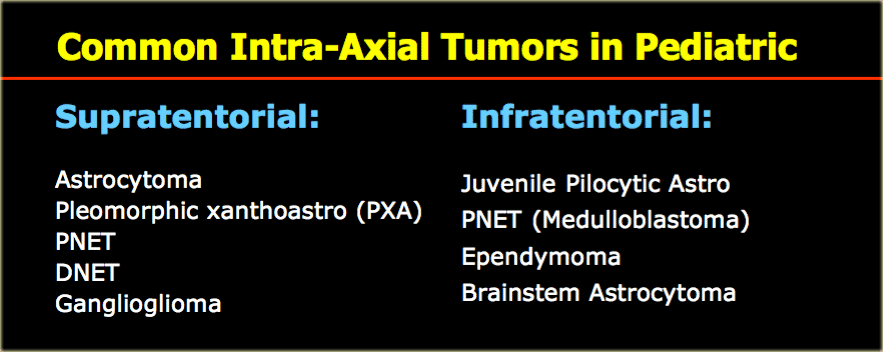
Although cancer is rare in children, brain tumors are the most common type of childhood cancer after leukemia and lymphoma.
Most of the tumors in children are located infratentorially.
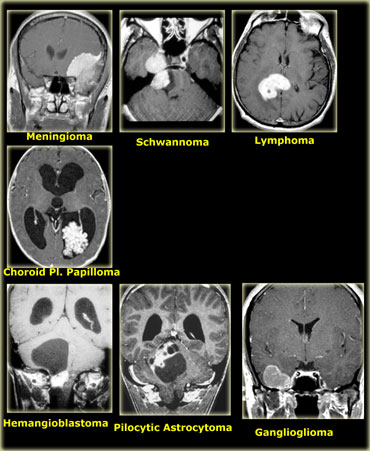
The most common supra- and infratentorial tumors are listed in the table on the left.
The most common tumors in adults are listed in the table on the left.
Note that metastases are by far the most common.
It is important to realize that 50% of metastases are solitary.
Particularly in the posterior fossa, metastases should be in the top 3 of the differential diagnostic list.
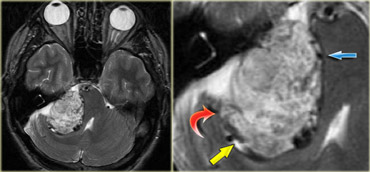
Hemangioblastoma is an uncommon tumor, but it is the most common primary intra-axial tumor in the adult.
Supratentorially, metastases are also the most common tumors, followed by gliomas.
Tumor spread
Intra- versus Extraaxial
When we study an intracranial mass, the first thing we want to know is whether the mass lies in- or outside of the brain.
If it is outside the brain or extra-axial, then the lesion is not actually a brain tumor, but derived from the lining of the brain or surrounding structures.
Eighty percent of these extra-axial lesions will be either a meningioma or a schwannoma.
On the other hand, in an adult an intra-axial tumor will be a metastasis or astrocytoma in 75% of cases.
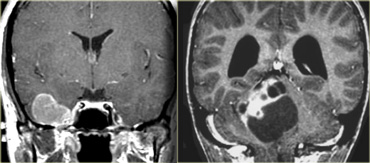
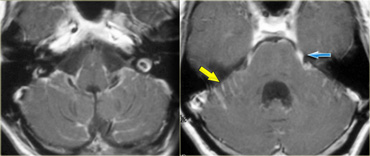
The T2W-images show a schwannoma located in the cerebellopontine angle (CPA).
This case nicely demonstrates the typical signs of an extra-axial tumor.
There is a CSF cleft (yellow arrow).
The subarachnoid vessels that run on the surface of the brain are displaced by the lesion (blue arrow).
There is gray matter between the lesion and the white matter (curved red arrow).
The subarachnoid space is widened because growth of an extra-axial lesion tends to push away the brain.
All these signs indicate that this is a typical extra-axial tumor.
In the region of the CPA 90% of the extra-axial tumors are schwannomas.
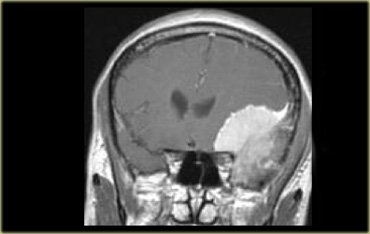 Coronal enhanced T1WI. Meningioma with dural tail, hyperostosis of adjacent bone and homogeneous enhancement
Coronal enhanced T1WI. Meningioma with dural tail, hyperostosis of adjacent bone and homogeneous enhancement
Another sign of an extra-axial origin is a broad dural base or a dural tail of enhancement as is typically seen in meningiomas.
This may also occur in other extra-axial tumors, but it is less common.
Another sign of an extra-axial origin are bony changes.
Bony changes are seen in bone tumors like chordomas, chondrosarcomas and metastases.
They can also be secondary, as is seen in meningiomas and other tumors.
On the left an example of a meningioma with a broad dural base and a dural tail of enhancement.
There is hyperostosis in the adjacent bone and the lesion enhances homogeneously.
Extra-axial tumors are not derived from brain tissue and do not have a blood-brain-barrier, so most of them enhance homogeneously.
Intra- vs Extra-axial (2)
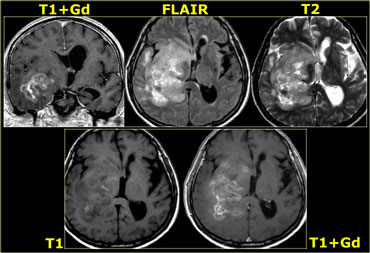
The differentiation between intra-axial versus extra-axial is usually straight forward, but sometimes it can be very difficult and imaging in multiple planes may be necessary.
The tumor in the case on the left was thought to be a falcine meningioma, i.e. extra-axial and was presented for surgery.
This lesion surely has the appearance of a meningioma: these tumors can be hypointense on T2 due to a fibrocollageneous matrix or calcifications and frequently produce reactive edema in the adjacent white matter of the brain.
However, there is gray matter on the anteromedial and posteromedial side of the lesion (red arrow).
This indicates that the lesion is intra-axial.
If the lesion was extra-axial the gray matter should have been pushed away.
This proved to be a melanoma metastasis.
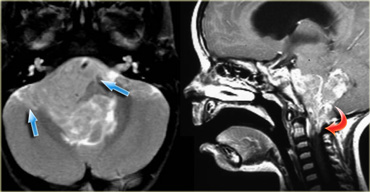 Ependymoma with extension to the prepontine area (blue arrows) and into the foramen magnum (red arrow).
Ependymoma with extension to the prepontine area (blue arrows) and into the foramen magnum (red arrow).
Local tumor spread (1)
Astrocytomas spread along the white matter tracts and do not respect the bounderies of the lobes.
Because of this infiltrative growth, in many cases the tumor is actually larger than can be depicted with MR.
Ependymomas of the fourth ventricle in children tend to extend through the foramen of Magendie to the cisterna magna and through the lateral foramina of Luschka to the cerebellopontine angle (figure).
Oligodendrogliomas typically show extension to the cortex.
Subarachnoid seeding
Some tumors show subarachnoid seeding and form tumoral nodules along the brain and spinal cord.
This is seen in PNET, ependymomas, GBMs, lymphomas, oligodendrogliomas and choroid plexus papillomas.
Primitive neuroectodermal tumours (PNET) form a rare group of tumors, which develop from primitive or undifferentiated nerve cells.
These include medulloblastomas and pineoblastomas.
One of the most important roles of imaging is to assess the extent of a tumor.
This is shown in the case on the left in a patient who presented with multiple cranial nerve abnormalities.
On the images we see an extra-axial tumor in the region of the left cavernous sinus.
There is homogeneous enhancement with a broad dural tail.
This is typical for a meningioma.
Only by studying all the images we do appreciate that the actual extent of the tumor is greater than expected.
The tumor is situated in the pterygopalatine fossa and extends into the orbit.
It also spreads anteriorly into the middle cranial fossa
Local tumor spread (2)
Another important consideration is the effect on the surrounding structures.
Primary brain tumors are derived from brain cells and often have less mass effect for their size than you would expect, due to their infiltrative growth.
This is not the case with metastases and extra-axial tumors like meningiomas or schwannomas, which have more mass effect due to their expansive growth.
On the left is an image of a diffusely infiltrating intra-axial tumor occupying most of the right hemisphere with only a minimal mass effect.
This is typical for the infiltrative growth seen in primary brain tumors.
There is no enhancement so this would probably be a low-grade astrocytoma.
Midline crossing
The ability of tumors to cross the midline limits the differential diagnosis.
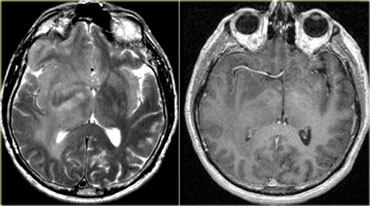
- Glioblastoma multiforme (GBM) frequently crosses the midline by infiltrating the white matter tracts of the corpus callosum.
- Radiation necrosis can look like recurrent GBM and can sometimes cross the midline.
- Meningioma is an extra-axial tumor and can spread along the meninges to the contralateral side.
- Lymphoma is usually located near the midline.
- Epidermoid cysts can cross the midline via the subarachnoid space.
- MS can also present as a mass lesion in the corpus callosum.
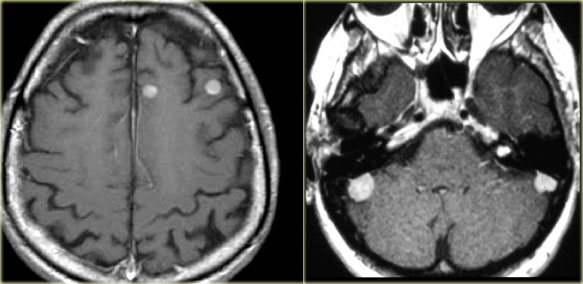 LEFT: Metastases. RIGHT: Multiple meningiomas and a schwannoma in a patient with Neurofibromatosis II
LEFT: Metastases. RIGHT: Multiple meningiomas and a schwannoma in a patient with Neurofibromatosis II
Multifocal disease
Multiple tumors in the brain usually indicate metastatic disease (figure).
Primary brain tumors are typically seen in a single region, but some brain tumors like lymphomas, multicentric glioblastomas and gliomatosis cerebri can be multifocal.
Some tumors can be multifocal as a result of seeding metastases: this can occur in medulloblastomas (PNET-MB), ependymomas, GBMs and oligodendrogliomas.
Meningiomas and schwannomas can be multiple, especially in neurofibromatosis type II.
Multiple brain tumors can be seen in phacomatoses:
- Neurofibromatosis I: optic gliomas and astrocytomas
- Neurofibromatosis II: meningiomas, ependymomas, choroid plexus papillomas (figure)
- Tuberous Sclerosis: subependymal tubers, intraventricular giant cell astrocytomas, ependymomas
- von Hippel Lindau: hemangioblastomas
Many non-tumorous diseases like small vessel disease, infections (septic emboli, abscesses) or demyelinating diseases like MS can also present as multifocal disease.
Cortical based tumors
Most intra-axial tumors are located in the white matter.
Some tumors, however, spread to or are located in the gray matter.
The differential diagnosis for these cortical based tumors includes oligodendroglioma, ganglioglioma and Dysembryoplastic Neuroepithial Tumor (DNET).
A DNET is a rare benign neoplasm, usually in a cortical and temporal location.
Patients with a cortically based tumor usually present with complex seizures.
On the left a 45-year-old female with a stable seizure disorder (complex-partial) for 15 years.
There is a non-enhancing, cortically based tumor.
This is a ganglioglioma.
The differential diagnosis includes DNET and pilocytic astrocytoma.
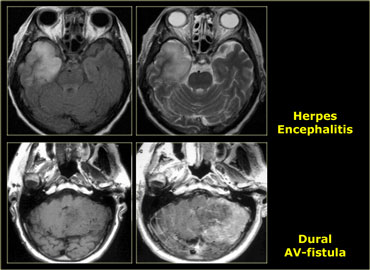
These cortically based tumors have to be differentiated from non-tumorous lesions like cerebritis, herpes simplex encephalitis, infarction and post-ictal changes.
On the left are images of a 52-year-old female who, over the period of one year, complained of headache and neck pain.
There is a recent onset of tonic-clonic seizures.
The CT shows a mass with calcifications, which extends all the way to the cortex.
Although this is a large tumor there is only limited mass effect on surrounding structures, which indicates that this is an infiltrating tumor.
The most likely diagnosis is oligodendroglioma.
The differential diagnosis includes a malignant astrocytoma or a glioblastoma.
CT and MR Characteristics
Fat - Calcification - Cyst - High density
Fat has a low density on CT (- 100HU).
On MR, fat has a high signal intensity on both T1- and T2WI.
On sequences with fat suppression fat can be differentiated from high signal caused by subacute hematoma, melanin, slow flow etc.
When you see high signal on T1WI always look for chemical shift artefact, as this indicates the presence of fat.
The chemical shift artefact occurs as alternating bands of high and low signal on the boundaries of a lesion and is seen only in the frequency encoding direction.
Fat within a tumor is seen in lipomas, dermoid cysts and teratomas.
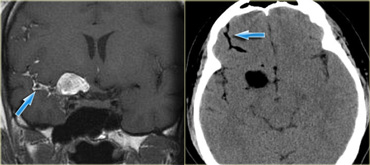
On the left a patient with the classical findings of a ruptured dermoid cyst.
Some tumors can have a high density on CT.
This is typically seen in lymphoma, colloid cyst and PNET-MB (medulloblastoma).
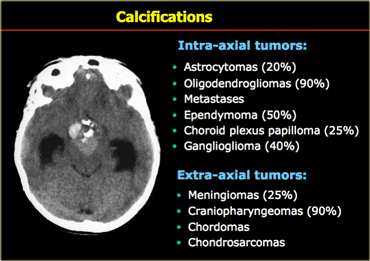
Calcification
Calcification is seen in many CNS tumors (Table).
When we think of a calcified intra-axial tumor, we think oligodendroglioma since these tumors nearly always have calcifications.
However an intraaxial calcified tumor in the brain is more likely to be an astrocytoma than a oligodendrogliomas, since astrocytomas, although less frequently calcified, are far more common.
A pineocytoma itself does not calcify, but instead it 'explodes' the calcifications of the pineal gland.
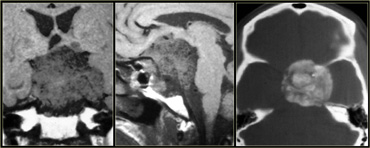
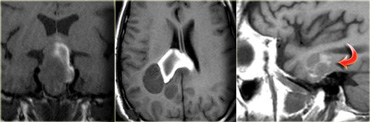
On the left is an image of a calcified mass in the suprasellar region, causing obstructive hydrocephalus.
This location in the suprasellar region and the calcification are typical for a craniopharyngioma.
Craniopharyngiomas are slow growing, extra-axial, squamous epithelial, calcified, cystic tumors arising from remnants of Rathke's cleft.
They are located the (supra)sellar region and primarily seen in children with a small second peak incidence in older adults.
On the left are images of a tumor with a small calcification. .
The calcification is not appreciated on the MR images, but is easily seen on CT.
The calcification and the extension of the tumor to the cortex are very typical for an oligodendroglioma.
An astrocytoma should be in the differential.
On the left are images of a patient with progressive visual loss.
On the coronal and sagittal TW1I there is a large mass centered around the sella with a broad dural base.
There is extension into the sella.
This patient was booked for decompression.
Only after the CT was performed, was it appreciated how densely calcified this tumor is.
It would be impossible to operate this tumor and preserve the patient's vision.
Cystic versus Solid
There are many cystic lesions that can simulate a CNS tumor.
These include epidermoid, dermoid, arachnoid, neuroenteric and neuroglial cysts.
Even enlarged perivascular spaces of Virchow Robin can simulate a tumor.
In order to determine whether a lesion is a cyst or cystic mass look for the following characteristics:
- Morphology
- Fluid/fluid level
- Content usually isointense to CSF on T1, T2 and FLAIR
- DWI: restricted diffusion
An arachnoid cyst is isointense to CSF on all sequences.
Tumor necrosis may sometimes look like a cyst, but it is never completely isointense to CSF.
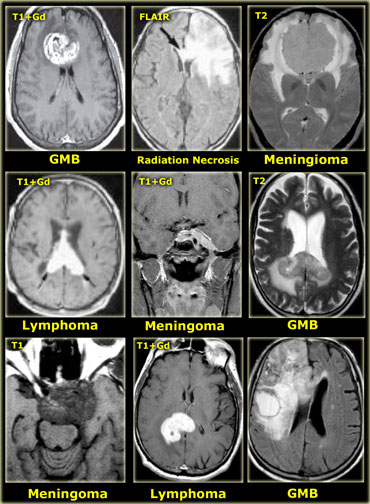
On the far left a craniopharyngioma with an enhancing rim surrounding the cystic component.
In the middle a neuroenteric cyst with the contents of which have the same signal intensity as CSF.
On the right a glioblastoma multiforme (GBM) with a central cystic component.
The enhancement in GBM is usually more irregular.
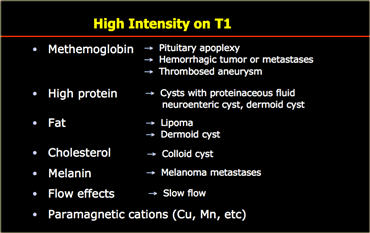
High on T1
Most tumors have a low or intermediate signal intensity on T1WI.
Exceptions to this rule can indicate a specific type of tumor.
On the left is a list of causes for T1-shortening.
Calcifications are mostly dark on T1WI, but depending on the matrix of the calcifications they can sometimes be bright on T1.
Especially on gradient echo images slow flow can be seen as bright signal on T1WI and should not be confused with enhancement.
This is particularly pronounced on gradient echo images.
If you only do an enhanced scan, remember that high signal is not always enhancement.
On the left are some images of tumors with high signal intensities on T1WI.
On the far left images of a patient who presented with apoplexy.
The high signal is due to hemorrhage in a pituitary macroadenoma.
The patient in the middle has a glioblastoma multiforme, which caused a hemorrhage in the splenium of the corpus callosum.
On the right is a patient with a metastasis of a melanoma.
The high signal intensity is due to the melanin content.
Low on T2
Most tumors will be bright on T2WI due to a high water content.
When tumors have a low water content they are very dense and hypercellular and the cells have a high nuclear-cytoplasmasmic ratio.
These tumors will be dark on T2WI.
The classic examples are CNS lymphoma and PNET (also hyperdense on CT).
Calcifications are mostly dark on T2WI.
The differential diagnosis of calcified tumors was discussed above.
Paramagnetic effects cause a signal drop and are seen in tumors that contain hemosiderin.
Proteinaceous material can be dark on T2 depending on the content of the protein itself.
A classic example of this is the colloid cyst.
Flow voids are also dark on T2 and indicate the presence of vessels or flow within a lesion.
This is seen in tumors that contain a lot of vessels like hemangioblastomas, but also in non-tumorous lesions like vascular malformations.
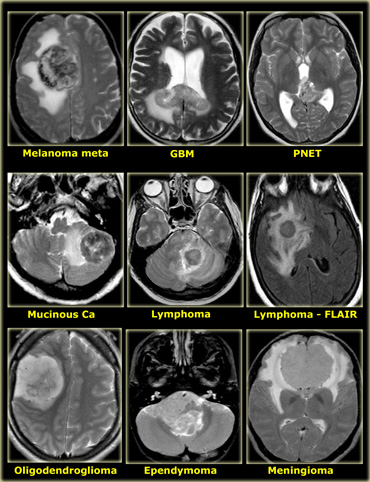
On the left some examples of tumors with a low signal intensity on T2WI.
- Melanoma metastases have a low SI on T2WI as a result of the melanin.
- GBM can have a low SI on T2WI because sometimes they have a high nuclear-cytoplasmic ratio. Most GBM's, however, are hyperintense on T2WI.
- PNET typically has a high nuclear-cytoplasmic ratio. PNET is mostly located in the region of the 4th ventricle, but another, less common, location is in the region of the pineal gland.
- Mucinous metastases can have a low SI on T2WI because they often contain calcifications..
- Meningiomas are mostly of intermediate signal.
They can have a high SI on T2WI if they contain a lot of water.
They can have a low SI on T2WI if they are very dense and hypercellular or when they contain calcifications.
Diffusion weighted imaging
Normally water protons have the ability to diffuse extracellularly and loose signal.
High intensity on DWI indicates restriction of the ability of water protons to diffuse extracellularly.
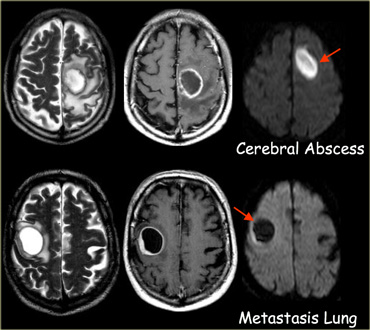
Restricted diffusion is seen in abscesses, epidermoid cysts and acute infarction (due to cytotoxic edema).
In cerebral abscesses the diffusion is probably restricted due to the viscosity of pus, resulting in a high signal on DWI.
In most tumors there is no restricted diffusion - even in necrotic or cystic components.
This results in a normal, low signal on DWI.
Perfusion Imaging
Perfusion imaging can play an important role in determining the malignancy grade of a CNS tumor.
Perfusion depends on the vascularity of a tumor and is not dependent on the breakdown of the blood-brain barrier.
The amount of perfusion shows a better correlation with the grade of malignancy of a tumor than the amount of contrast enhancement.
Enhancement
Blood brain barrier
The brain has a unique triple layered blood-brain barrier (BBB) with tight endothelial junctions in order to maintain a consistent internal milieu.
Contrast will not leak into the brain unless this barrier is damaged.
Enhancement is seen when a CNS tumor destroys the BBB.
Extra-axial tumors such as meningiomas and schwannomas are not derived from brain cells and do not have a blood-brain barrier.
Therefore they will enhance.
There is also no blood-brain barrier in the pituitary, pineal and choroid plexus regions.
Some non-tumoral lesions enhance because they can also break down the BBB and may simulate a brain tumor.
These lesions include like infections, demyelinating diseases (MS) and infarctions.
Contrast enhancement cannot visualize the full extent of a tumor in cases of infiltrating tumors, like gliomas.
The reason for this is that tumor cells blend with the normal brain parenchyma where the blood brain barrier is still intact.
Tumor cells can be found beyond the enhancing margins of the tumor and beyond any MR signal alteration - even beyond the area of edema.
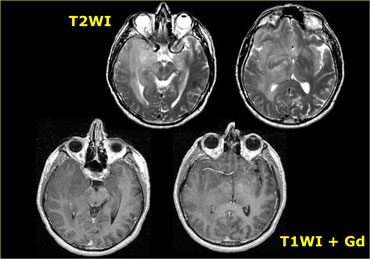
On the left is an image of a 42 y/o male with mild head trauma.
On the T2WI there is a lesion in the left temporal lobe, found incidentally.
There was no enhancement and the DWI was normal.
During follow-up there was a slight increase in size.
This was diagnosed as a low-grade astrocytoma.
It is not possible to resect such a lesion, since the infiltrating tumors cells are within the normal-appearing brain tissue.
In gliomas - like astrocytomas, oligodendrogliomas and glioblastoma multiforme - enhancement usually indicates a higher degree of malignancy.
Therefore when during the follow up of a low-grade glioma the tumor starts to enhance, it is a sign of malignant transformation..
Gangliogliomas and pilocytic astrocytomas are the exceptions to this rule: they are low-grade tumors, but they enhance vividly.
As discussed above, it recently has been shown that tumor angiogenesis as shown by perfusion MR correlates better with tumor grade than enhancement after the administration of intravenous contrast.
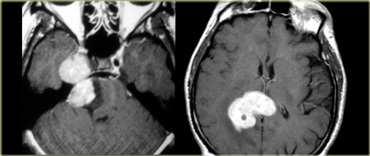 LEFT: Schwannoma extending into the middle cranial fossa with homogeneous enhancement RIGHT: Primary Lymphoma known for its vivid enhancement
LEFT: Schwannoma extending into the middle cranial fossa with homogeneous enhancement RIGHT: Primary Lymphoma known for its vivid enhancement
The amount of enhancement depends on the amount of contrast that is delivered to the interstitium.
In general, the longer we wait, the better the interstitial enhancement will be.
The optimal timing is about 30 minutes and it is better to give contrast at the start of the examination and to do the enhanced T1WI at the end.
No enhancement is seen in:
- Low grade astrocytomas
- Cystic non-tumoral lesions:
- Dermoid cyst
- Epidermoid cyst
- Arachnoid cyst
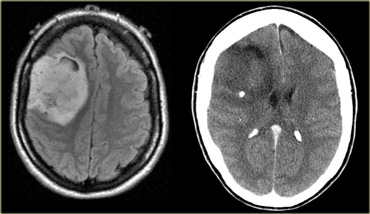
On the left is an image of an intra-axial tumor in an adult.
It is centered in the temporal lobe and involves the cortex.
Although there is massive infiltrative growth involving a large part of the right cerebral hemisphere, there is only minimal mass effect.
There is no enhancement.
These features are typical for a low-grade astrocytoma.
Homogeneous enhancement can be seen in:
- Metastases
- Lymphoma
- Germinoma and other pineal gland tumors
- Pituitary macroadenoma
- Pilocytic astrocytoma and hemangioblastoma (only the solid component)
- Ganglioglioma
- Meningioma and Schwannoma
Patchy enhancement can be seen in:
- Metastases
- Oligodendroglioma
- Glioblastoma multiforme
- Radiation necrosis
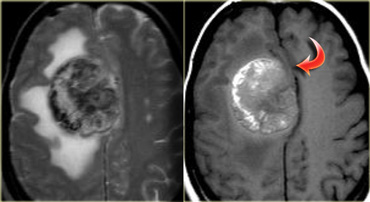
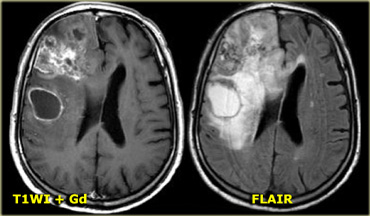
On the left is an example of a glioblastoma multiforme (GBM).
The enhancement indicates that this is a high-grade tumor, but only parts of it enhance.
Notice that there is also a cystic component with ring enhancement.
The tumor cells probably extend beyond the area of edema as seen on the FLAIR image.
This is because gliomas grow infiltratively into normal brain - initially without any MR changes.
Patchy enhancement (2)
On the left are images of a tumor located in the right hemisphere.
Although is a large tumor, the mass-effect is limited.
This indicates that there is marked infiltrative growth, a characteristic typical for gliomas.
Notice the heterogeneity on both T2WI and FLAIR.
There is patchy enhancement.
All these findings are typical for a GBM.
Virtually no other tumor behaves in this way.
Ring enhancement
Ring enhancement is seen in metastases and high-grade gliomas.
It is also seen in non-tumorous lesions like abscesses, some MS-plaques and sometimes in an old hematomas.
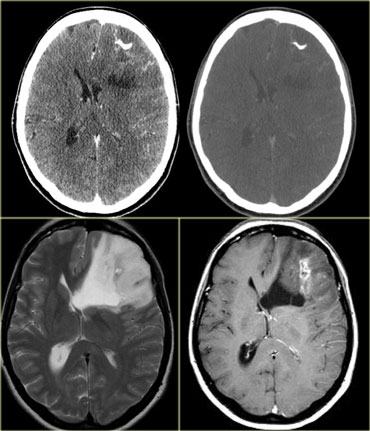
On the left three different ring enhancing lesions.
Conspicuity of tumors with contrast
The case on the left demonstrates the value of Gadolinium in the conspicuity of tumors.
This is a patient with Neurofibromatosis II.
After the administration of contrast the two meningiomas and the schwannoma are easily seen.
Leptomeningeal metastases are usually not seen without the administration of intravenous contrast.
The case on the left demonstrates the abnormal enhancement along the brainstem, along the folia of the cerebellum (yellow arrow) and along the fifth intracranial nerve (blue arrow) in a patient with leptomeningeal metastases.
Differential diagnosis for specific anatomic area
Skull base
Common skull base tumors are listed in the table on the left.
These tumors either arise from extracranial structures like the sinuses (sinonasal carcinoma), or from the skull base itself (chordoma, chondrosarcoma, fibrous dysplasia).
Chordoma is usually located in the midline, while chondrasarcoma usually arises off the midline.
On the left a midline tumor arising from the clivus.
This is the typical presentation of a chordoma.
The differential diagnosis would include a metastasis and a chondrosarcoma.
On the left another skull base tumor located off midline.
This is a typical presentation for a chondrosarcoma.
The differential diagnosis would include a metastasis and a paraganglioma.
Chondrosarcomas can be located in the midline and chordomas are sometimes located off midline but those cases are exceptional.
On the left an example of a Skull Base Paraganglioma.
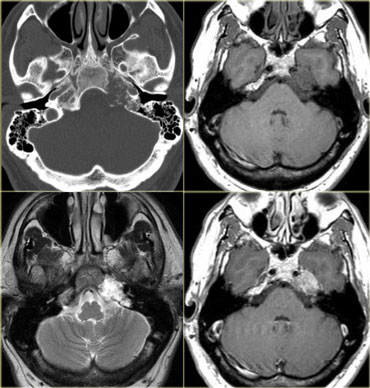
On the left CT images of a 58-year-old male with a gradual onset of right facial pain and numbness and a recent onset of double vision.
First study the images, than continue.
There is an enhancing mass anterior to the skull base and also in the region of the right cavernous sinus.
In the bone window setting there is sclerosis of the skull base, particularly in the region of the clivus.
Continue with the MR images.
On the left enhanced sagittal and coronal T1WI.
The most striking finding is the black clivus due to the sclerosis.
A normal clivus is bright on T1WI as a result of the fatty bone marrow.
There is an enhancing mass anterior to the clivus.
On the coronal images we see the enhancement extending through the foramen ovale to the right of the cavernous sinus.
The diagnosis is a nasopharyngeal squamous cell carcinoma with intracranial extension.
The differential diagnosis would include: skull base metastasis, lymphoma, chronic infection and even a meningioma - although this would be an unusual way for a meningioma to spread.
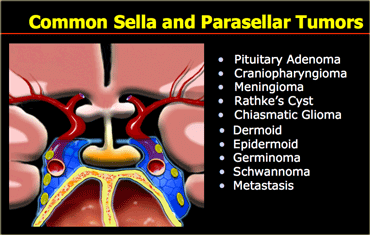
Sella/suprasellar
On the left is a list of common sellar and suprasellar tumors.
In this region it is important to keep the possibility of an aneurysm in the differential diagnosis.
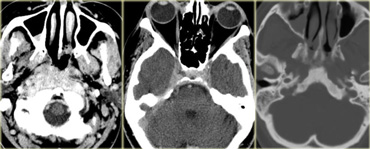
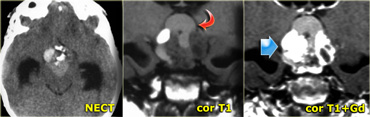
On the left are images of a mass in the suprasellar cistern.
On the NECT we can see that it contains calcium.
On the T1WI there is a hyperintense area that shows no enhancement (i.e. cystic).
There are other components that show enhancement.
The tumor is complicated by a hydrocephalus.
These findings are very specific for a craniopharyngeoma.
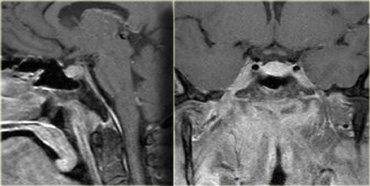
On the left NECT and enhanced CT-images of a 33-year-old female with severe headache (worse in the a.m.), reduction in visual acuity and visual fields and papilledema.
Continue with the MR images.
Notice the normal inferiorly displaced pituitary gland.
This means it is not a macroadenoma.
The diagnosis is again a craniopharyngioma.
The differential diagnosis would include an astrocytoma and a meningioma.
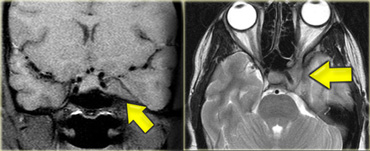
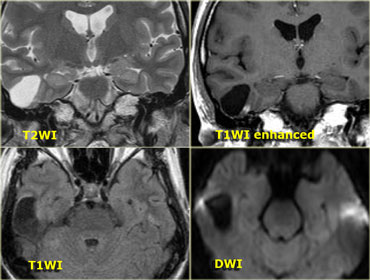
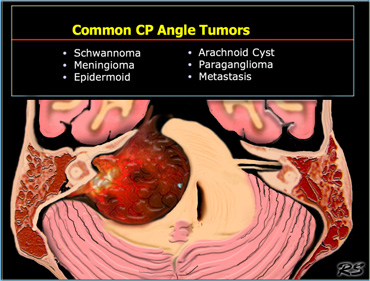
Cerebello-pontine angle
Common CP Angle Tumors are listed in the table on the left.
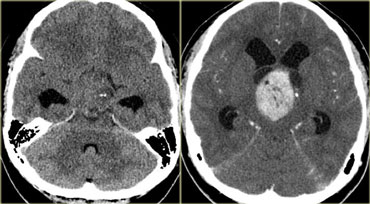
On the left a 52-year-old male with hearing loss on the right.
The images show an unusual cystic mass with enhancing septations.
There is also some enhancement within the internal acoustic canal.
Based on the images the most likely diagnosis would be a cystic schwannoma, but this happened to be an uncommon, cystic presentation of a meningioma.
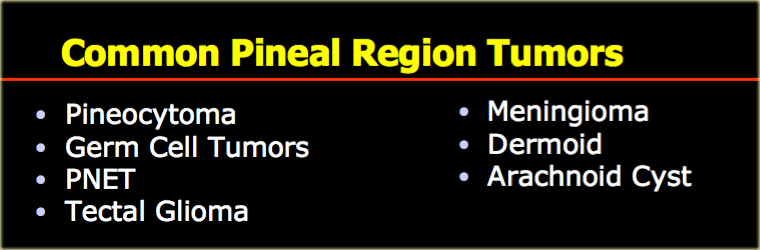
Pineal region
Common pineal region tumors are listed in the table on the left.
On the left a tumor located in the pineal region.
Based on these images the differential diagnosis would include:
- Meningioma
- Pineocytoma
- Germ Cell Tumor
This happened to be a meningioma.
On the left are typical images of a ruptured pineal region dermoid.
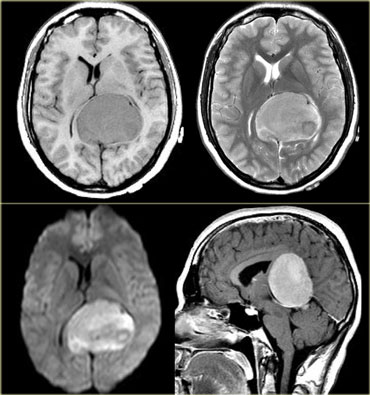
On the left images of a 12 y/o male with upward gaze paralysis.
There is a tumor located in the pineal region.
The tumor contains calcifications.
There is homogeneous enhancement, which is common for a tumor in the pineal region (discussed above).
Based on the age of the patient, the location and the tumor characteristics, this is most likely a germinoma.
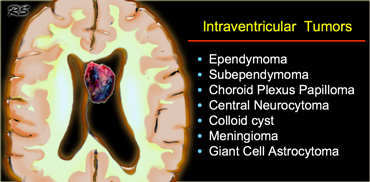
Intraventricular
Common intraventricular Tumors are listed in the table on the left.
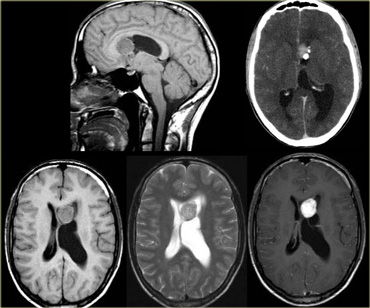
On the left a tumor located in the 3rd ventricle.
The tumor contains calcifications.
The diagnosis is a giant cell astrocytoma.
4th ventricle
In children tumors in the 4th ventricle are very common.
Astrocytomas are the most common followed by medulloblastomas (or PNET-MB), ependymomas and brainstem gliomas with a dorsal exophytic component.
In adults tumors in the 4th ventricle are uncommon.
Metastases are most frequently seen, followed by hemangioblastomas, choroid plexus papillomas and dermoid and epidermoid cysts.
Tumor Mimics
Many non-tumorous lesions can mimic a brain tumor.
Abscesses can mimic metastases.
Multiple sclerosis can present with a mass-like lesion with enhancement, also known as tumefactive multiple sclerosis..
In the parasellar region one should always consider the possibility of a aneurysm.
Infections and vascular lesions can also mimic a CNS tumor.
Charity
All the profits of the Radiology Assistant go to Medical Action Myanmar which is run by Dr. Nini Tun and Dr. Frank Smithuis sr, who is a professor at Oxford university and happens to be the brother of Robin Smithuis.
Click on the image below to watch the video of Medical Action Myanmar and if you like the Radiology Assistant, please support Medical Action Myanmar with a small gift.