Rectal Cancer MR staging 3.0
Doenja Lambregts, Rhiannon van Loenhout, Frank Zijta, Max Lahaye, Regina Beets-Tan and Robin Smithuis
Radiology Departement of the Netherlands Cancer Institute in Amsterdam, the Medical Centre Haaglanden in the Hague and the Alrijne Hospital in Leiderdorp, the Netherlands
Publicationdate
This is the third version describing the role of MRI for the staging and restaging of rectal cancer.
The two major advancements in the treatment of rectal cancer are total mesorectal excision (TME) and neoadjuvant radiotherapy with or without chemotherapy.
Both have dramatically changed the local recurrence and survival rates.
MRI is the most accurate tool for the local staging of rectal cancer and is a powerful tool to select the appropriate treatment.
The decision whether a patient with rectal cancer is a candidate for TME only or neoadjuvant therapy followed by TME, is made on the findings of the MRI.
This updated version includes:
- An overview of the main pitfalls when reading and reporting MRI of rectal cancer.
- Reporting templates for primary staging and restaging.
- Use of DWI in restaging.
- TNM staging 8th edition.
- Definition of rectosigmoid junction and lateral lymph nodes.
- A brief overview of current treatment in rectal cancer, including organ preservation
In the end section there are two videos on how to report rectal cancer according to the structured reporting checklist.
If you first want to look at the videos click here.
Introduction
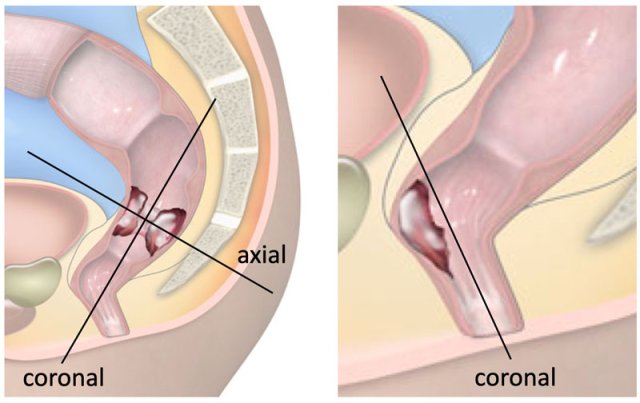
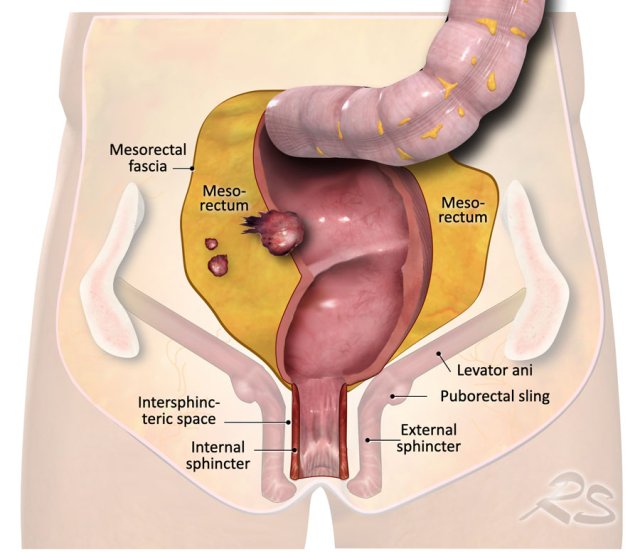 The illustration shows the mesorectum and the mesorectal fascia, which is the plane for TME resection and the relation of the rectum to the anal sphincter and pelvic floor.
The illustration shows the mesorectum and the mesorectal fascia, which is the plane for TME resection and the relation of the rectum to the anal sphincter and pelvic floor.
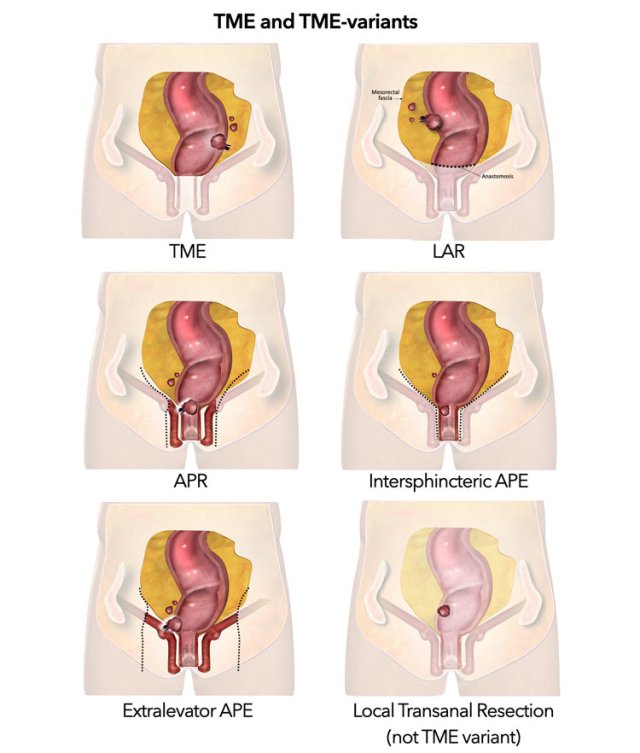
Total Mesorectal Excision
In TME the entire mesorectal compartment including the rectum, surrounding mesorectal fat and perirectal lymph nodes are completely removed along the mesorectal fascia (figure).
TME is the standard surgical resection technique for rectal cancer and can be performed as either a low anterior resection (LAR) where the anal canal is left in situ or an abdominoperineal resection (APR), where both the rectum and anal canal are resected.
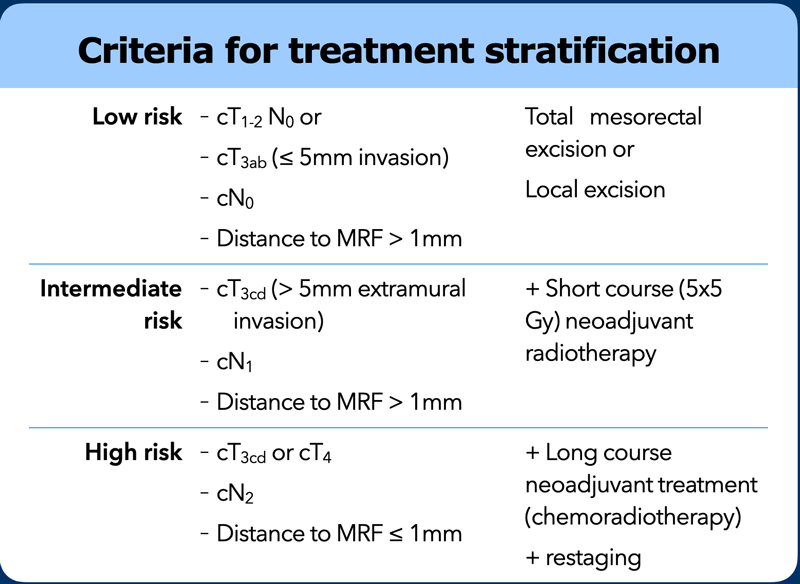
Risk Stratification
Local staging with MRI is performed to determine the best surgical strategy and the necessity for neoadjuvant treatment.
Criteria used to define low risk, intermediate risk and high risk disease and their use for treatment stratification vary between countries and guidelines and are continuously evolving. As a rule of thumb we can state the following:
Low Risk
T1, T2 and in several countries also early stage T3ab tumors without evidence of nodal metastases will generally be considered low risk and do not necessarily require neoadjuvant treatment.
Intermediate Risk
More extensive T3 tumors and/or tumors with a limited number of suspicious nodes are often considered intermediate risk. A short course of radiotherapy (5 x 5 Gy) prior to TME has been shown to reduce the local recurrence risk for these intermediate risk tumors [ref].
High risk or Locally advanced
Advanced T3-4 tumors that invade the mesorectal fascia or adjacent organs, or tumors with many suspicious nodes (N2) are typically regarded as locally advanced. These generally receive a long course of combined chemoradiotherapy aiming to induce tumor downsizing and downstaging and enhance the chance of a complete surgical resection [ref].
More recently, the presence of extramural vascular (or venous) invasion has been proposed as an additional adverse prognostic feature that should be considered a sign of high risk disease.
There is a growing tendency to consider minimally invasive or non-surgical treatment alternatives in tumors that show a complete or near-complete response after neoadjuvant treatment.
MRI plays an important role in addition to endoscopy to help select the right patients for these “organ preserving” treatments.
In addition to organ-preservation, recent advances in treatment include:
- development of new neoadjuvant treatment schemes such as combinations of prolonged neoadjuvant chemotherapy with short-course radiotherapy
- radiotherapy boost strategies
- immunotherapy
- evolutions in local excision and local radiotherapy techniques for early stage tumors or small tumor remnants after neoadjuvant treatment.
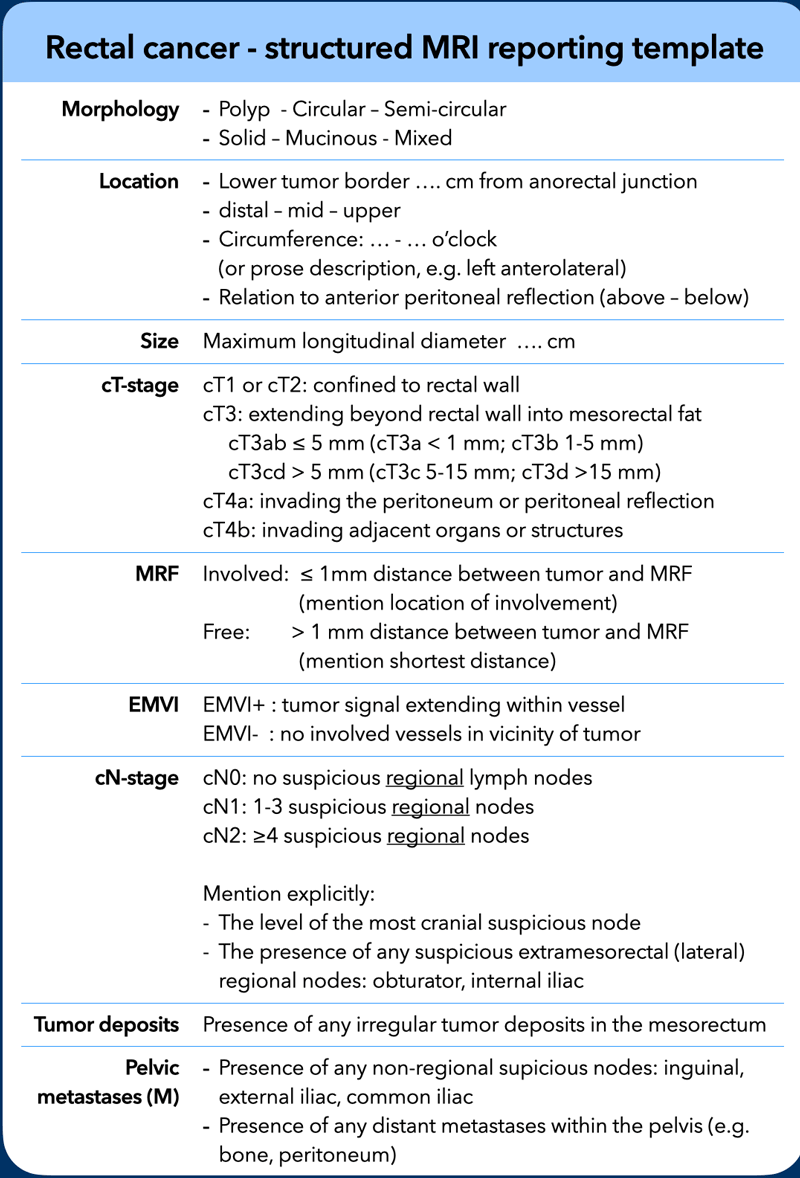
Structured Reporting Checklist
A good quality MRI report includes all risk factors used to stratify patients into differentiated treatments, as well as an accurate description of the relation of the tumor to its surrounding anatomy to inform surgical planning (figure).
In the following chapters we will discuss these various items listed in the reporting template in further detail
TNM-prefixes
“c” is used to indicate the clinical stage, determined before treatment. When defined based on imaging, the prefix “i" (imaging) or “mr” (MRI) are sometimes used as alternatives.
“y” is used to restage tumors after neoadjuvant treatment (chemo and/or radiotherapy) and can be used for both clinical staging (ycTNM) as well as pathological staging (ypTNM).
“p” indicates the final TNM stage as determined at histopathology after surgery.
Morphology
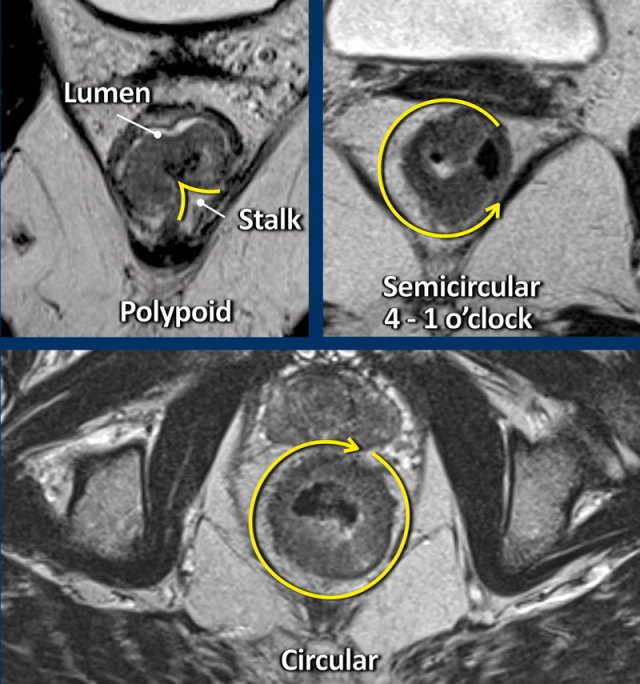
Polypoid and Sessile tumors
Rectal adenocarcinomas typically arise from adenomas that can be either polypoid, which are tumors raised upon a stalk or sessile, which are flat.
Polypoid tumors generally represent more low-grade malignancies and present as a mass projecting into the bowel lumen with a focal attachment or stalk to the bowel wall.
Sessile tumors typically present as a semi-circular or circular wall thickening.
The site where the tumor is attached to the rectal wall is often referred to as the “invasive margin” and is the site where we need to focus on when assessing the T-stage and looking for extramural tumor extension.
The degree of attachment to the rectal wall, also referred to as the tumor circumference, can be described in the radiology report as “from … to … o’clock”, or alternatively using prose descriptions such as “left anterolateral”.
Solid and Mucinous
The
distinction between solid and mucinous tumor types is relevant because mucinous
adenocarcinomas have a poorer prognosis and
typically show a poorer response to neoadjuvant treatment.
Mucinous tumors show distinct bright signal on T2-weighted MRI compared to the more intermediate signal of solid type tumors (figure).
A
more rare subtype of rectal cancer is the signet-ring cell carcinoma, which is
associated with a high risk for nodal and distant metastases and poor overall
survival.
It is seen in only approximately one percent of cases.
On MRI signet-ring cell carcinomas can be difficult to
discern, though they typically show long-segment diffuse bowel wall thickening
and a submucosal growth pattern that results in a ‘target’ appearance on axial
images.
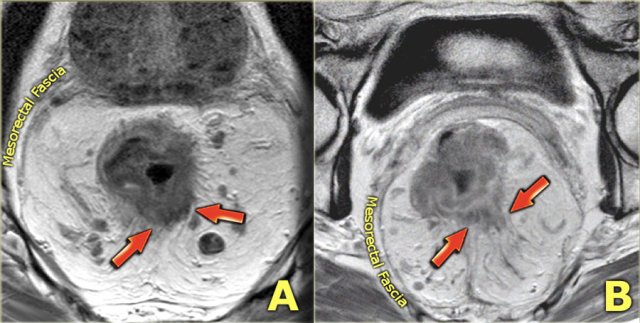
The images show a signet-ring cell carcinoma with diffuse thickening of the rectal wall and the target appearance on the axial image. Also note the diffuse infiltration of the mesorectal fat, which is another common finding of signet ring tumors.
Location
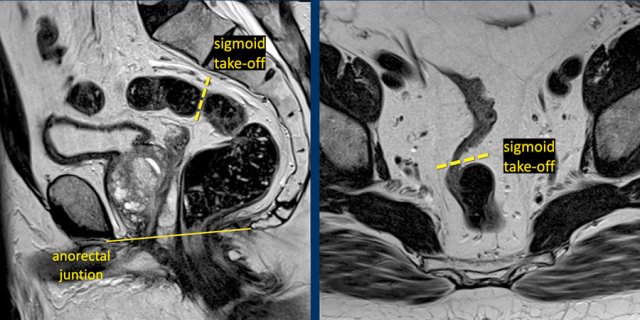
Sigmoid take-off
Discriminating rectal from sigmoid cancer is important because the treatment approach differs considerably. Routine treatment for sigmoid cancer is upfront resection, while rectal tumours undergo differentiated treatments varying from surgery only in low risk tumors to long course neoadjuvant chemoradiotherapy in high risk tumors.
In 2019 an international consensus panel agreed on the “sigmoid take-off” as the preferred landmark to define the rectosigmoid junction and separate the rectum from the sigmoid on imaging (ref).
The sigmoid take off can be recognized on sagittal MRI as the point from which the sigmoid sweeps horizontally away from the sacrum.
The sigmoid take-off can be recognized on sagittal MRI as the point from which the sigmoid sweeps horizontally away from the sacrum and on an axial view as the point from which the sigmoid projects ventrally (figure).
Though recognizing the sigmoid take-off on imaging may be challenging in some cases due to anatomical variations between patients or varying sequence angulation, it is overall an intuitive landmark.
Tumors above the level of the rectosigmoid junction with a lower border starting proximal to the sigmoid take-off are considered sigmoid tumors.
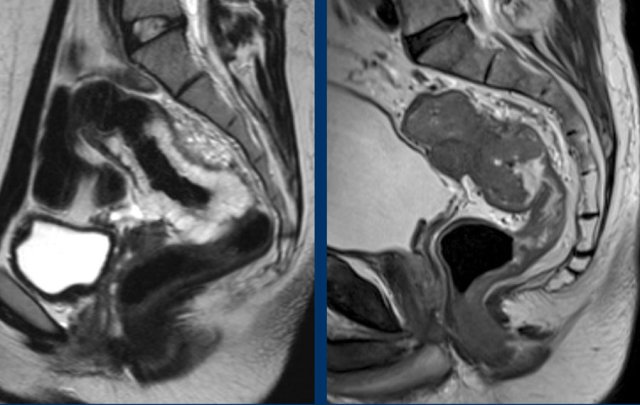
Tumor height
The
anorectal junction marks the transition between the anal canal and
distal rectum.
It is situated at the level of the anorectal angle, which is
caused by contraction of the puborectalis muscle.
On sagittal MRI the anorectal
junction is typically situated at the level of an imaginary line between the
lower margin of the sacral bone and pubic bone.
A common approach to determine the height of rectal tumors is to measure the distance between the lower margin of the tumor and the anorectal junction, or alternatively the distance from the anal verge, which is the transition between the anal epithelium and perianal skin.
In some countries such as the US the location of the tumor in relation to the anterior peritoneal reflection is commonly used as a landmark to determine the tumor height.
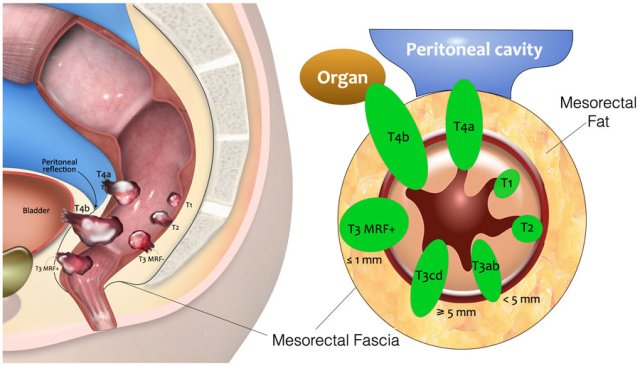
T-stage
This illustration shows the T-stages in the sagittal and axial plane.
- T1 tumors only invade the submucosa.
- T2 tumors invade the muscularis propria, which is the outer muscular layer of the rectal wall.
- T3 tumors extend into the mesorectal fat.
- T3 MRF- tumors do not reach the mesorectal fascia (MRF).
- T3 MRF+ tumors invade the mesorectal fascia or have a margin of ≤1mm from the mesorectal fascia
- T4a tumors invade the peritoneum or peritoneal reflection.
- T4b tumors invade other organs or structures outside the mesorectum.
The table shows an overview of the T-staging.
Low risk T3 tumors have minimal extension into the mesorectal fat up to 5mm (T3ab) and the mesorectal fascia is not involved (distance ≥1mm).
High risk T3 tumors extend into the mesorectal fat by > 5mm or invade the mesorectal fascia.
T4b tumors invade other organs or structures that are situated outside the mesorectum. The TNM classification system does not include a clear description of what is covered by the umbrella term “structures”.
In an international multidisciplinary consensus meeting from 2021 an expert panel of radiologists, surgeons, radiation oncologists and pathologists proposed that T4b should include invasion of the structures as mentioned in the table [ref].
T1-T2 – limited to the bowel wall
T1
and T2 tumors are limited to the bowel wall and have a relatively good
prognosis.
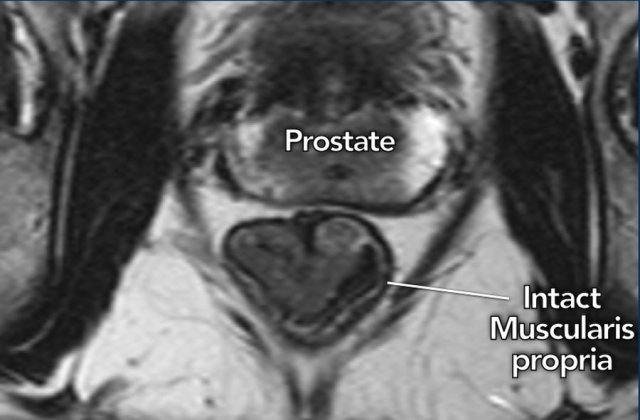
The key finding to ensure that a tumor remains limited to the
bowel wall (T1-2) is the presence of an intact muscularis propria, which can be
recognized on MRI as an intact hypointense line surrounding the rectum.
Image
A cT1-2 tumor in the distal rectum is shown with an intact muscularis
propria, which is clearly recognizable as a hypointense outer line of the
rectal wall.
Pitfall: differentiating T1 from T2
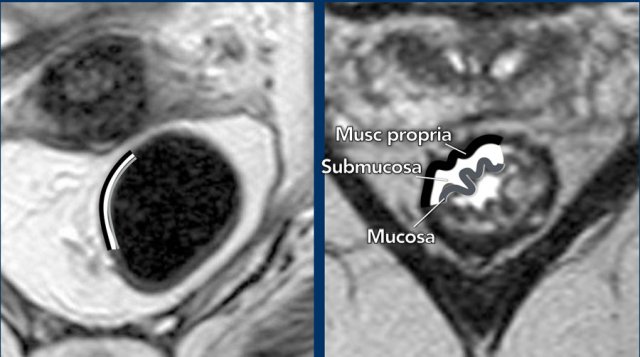
Anatomically,
the rectal wall is comprised of 3 main layers:
- Inner mucosal layer with intermediate signal on T2-weighted images,
- Middle submucosal layer with high signal on T2-weighted images
- Outer muscularis propria with low
signal on T2-weighted images.
Typically,
these 3 separate layers on MRI can only be recognized in the case of submucosal
edema.
In the absence of edema
the rectal wall generally has a two-layered appearance where we can recognize
the muscularis propria but cannot distinguish the mucosa from the submucosa.
This is the reason why MRI is generally unable to distinguish T1 tumors (growing into the submucosa) from T2 tumors (growing into the muscularis propria).
Endorectal ultrasound is more accurate for this purpose. Tumors that remain limited to the rectal wall are therefore typically grouped as cT1-2 on MRI.
T3 – invasion into the mesorectal fat
T3-tumors
grow through the muscularis propria into the surrounding mesorectum.
On MRI this can be recognized as an interruption of the
hypointense muscularis propria with spicular or nodular extension of tumor
signal beyond the rectal wall into the mesorectal fat.
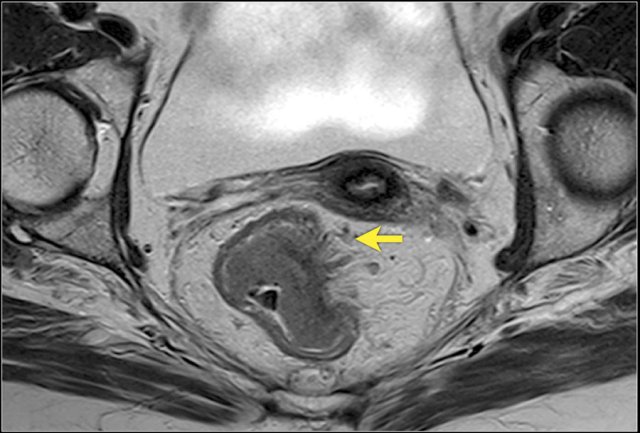
Image
A semicircular rectum tumor with invasion into the mesorectum from
approximately 1 to 4 o’clock. It does not grow within 1mm of the mesorectal
fascia.
The T-stage is T3 MRF- rectal cancer.
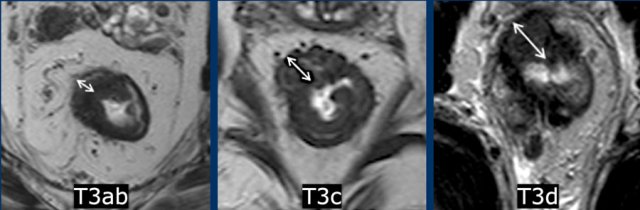
Subclassification
of T3 stage according to invasion depth:
Low-risk T3-tumors:
- T3a: tumor extends <1 mm beyond muscularis propria
- T3b: tumor extends 1-5 mm beyond muscularis propria
High-risk T3-tumors:
- T3c: tumor extends 5-15 mm beyond muscularis propria
- T3d: tumor extends > 15 mm beyond muscularis propria
- T3 MRF+ tumor ≤ 1mm of the MRF
Pitfall: perirectal stranding
It can be difficult to discern true perirectal tumor invasion in T3 tumors (case A) from desmoplastic stranding in T1-2 tumors (case B), which can be a potential cause of overstaging.
Note:
The clinical significance
to discern T2 from borderline T3 tumors has been argued as various current guidelines – including the Dutch guidelines –
classify T3 tumors with limited extension into the mesorectal fat (cT3ab) in
the same good prognostic group as T2 tumors for treatment stratification.
In some guidelines, however, T3 disease by itself is still considered a factor used to determine the need for neoadjuvant treatment.
Mesorectal fascia involvement
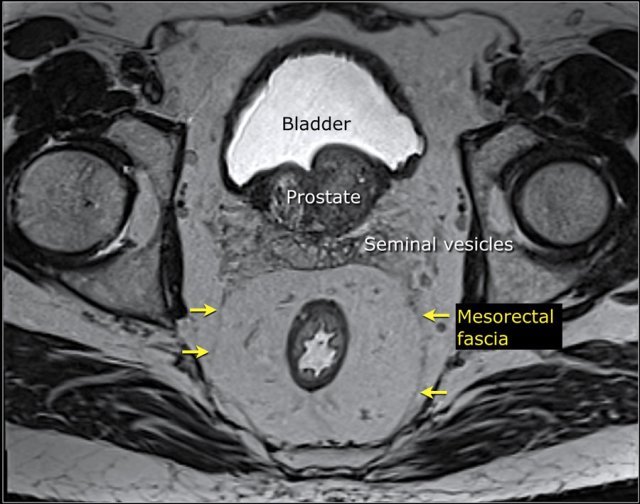
The mesorectal fascia (MRF) is a thin fibrous structure that encloses the mesorectal compartment and comprises the anticipated resection plane in TME surgery.
On T2-weighted MRI the mesorectal fascia can be recognized as a thin hypointense line surrounding the mesorectum.
When a tumor directly invades the MRF or the margin between the tumor and MRF is ≤ 1 mm, the MRF is involved.
In these cases routine TME would induce a risk for local recurrence and neoadjuvant treatment will be required to induce tumor downsizing to retract the tumor from the MRF aiming to achieve a tumor-free resection margin.
When describing involvement of the MRF, you should always describe the location of involvement (e.g., “MRF+ at … o’clock” or “MRF+ at the left anterior side”)
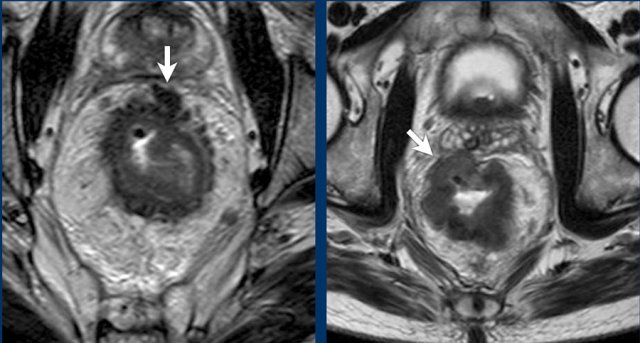 Two examples of T3 tumors with invasion of the mesorectal fascia. In the left case the distance between the tumor and the MRF is less than 1 mm at 12 o’clock. In the right case there is more extensive involvement of the MRF between 10 and 12 o’clock
Two examples of T3 tumors with invasion of the mesorectal fascia. In the left case the distance between the tumor and the MRF is less than 1 mm at 12 o’clock. In the right case there is more extensive involvement of the MRF between 10 and 12 o’clock
Pitfall: circumferential resection margin
A radiology report for T3 tumors should include a description of the smallest distance between the tumor and the MRF, which is sometimes alternatively referred to in radiological reports as the ‘circumferential resection margin’ (CRM).
This use of CRM as a synonym for MRF is not fully accurate as the CRM is actually the margin that the surgeon creates when performing a TME.
Ideally, this will be along the MRF, but the CRM may be smaller when the MRF is breached during surgery or wider when the TME resection specimen includes additional fat outside the MRF.
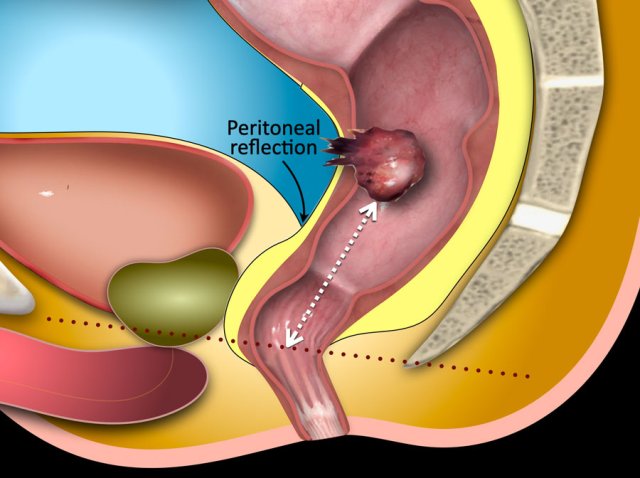
Mesorectal fascia versus Peritoneum
The low rectum is totally covered by the mesorectal fascia (green line).
In the mid rectum the mesorectum is covered by the mesorectal fascia on the posterior and lateral side, but on the anterior side it is covered by the visceral peritoneum (red line indicating the peritoneal reflection).
In the high rectum the peritoneal lining extends from the anterior to the lateral side (yellow line) and the MRF only lines the dorsal part of the mesorectum.
This distinction is important because invasion of the MRF constitutes T3 MRF+ disease, while growth into the visceral peritoneum entails a risk for tumor spread into the peritoneal cavity and is staged as T4a disease.
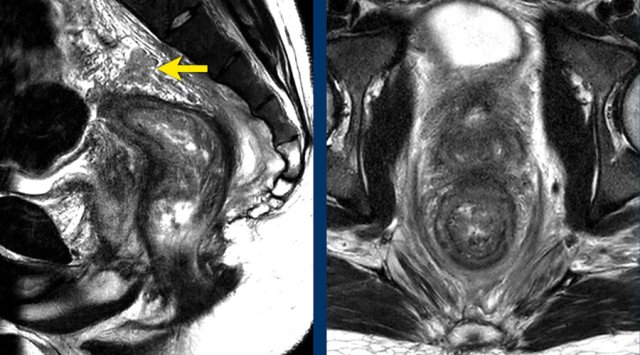
T4a - Invasion of peritoneum or peritoneal reflection
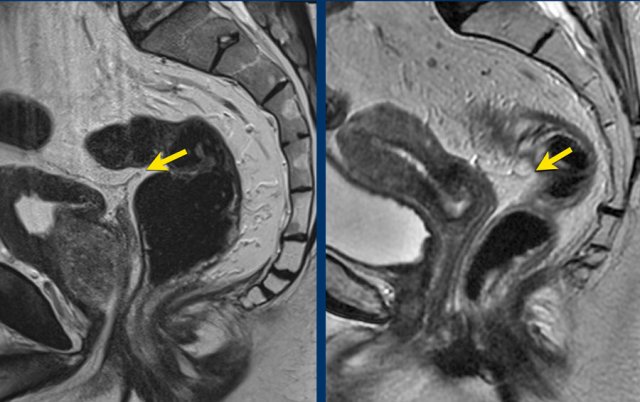
The
anterior peritoneal reflection marks the transition between the
non-peritonealized and peritonealized portions of the rectum.
On sagittal
T2-weighted images the peritoneal reflection can be recognized as a hypointense
V-shaped thin line, sometimes referred to as the ‘seagull sign’.
In
males it is located just above the seminal vesicles.
In females it is located at the level of the cul-de-sac
(Douglas).
Pitfall: Overstaging of upper rectal tumours:
In upper rectal tumors there will often be a close margin between the rectum and peritoneum.
This does not necessarily mean that we are dealing with a T4a tumor.
Tumors should only be classified as cT4a if there is clear tumor extension into or beyond the peritoneum or peritoneal reflection
Images
The image on the left shows a tumor with a close relation to the peritoneum and the bladder (white arrow).
However there is no tumor extension beyond the muscularis propria anteriorly and the peritoneum is therefore not invaded.
The image on the right shows definite tumor invasion of the peritoneum (yellow arrow), i.e. a T4a tumor.
Pitfall: reporting of MRF versus peritoneal invasion
Note
that in anterior tumors, MRF invasion can only occur in tumors below the
peritoneal reflection.
Tumors above the peritoneal reflection that invade the
peritoneum anteriorly (i.e. T4a tumours) are sometimes erroneously reported as
MRF+ tumors, which is not correct.
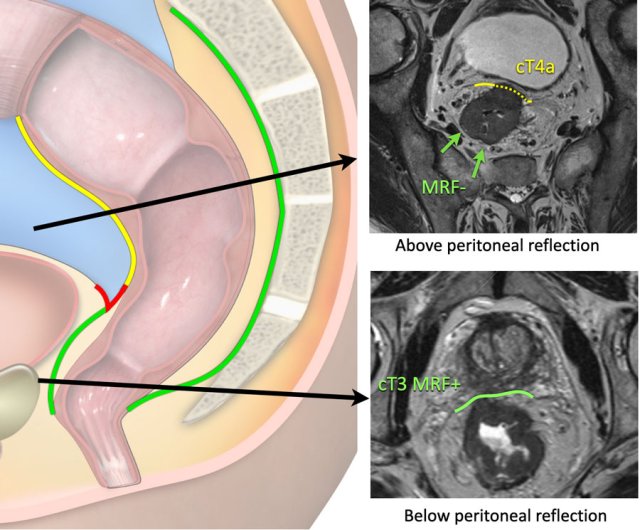
cT4a MRF-
The upper image shows a cT4a tumor in the upper rectum above
the level of the peritoneal reflection.
There is involvement of the peritoneum (yellow stippled line), but not of the
mesorectal fascia.
cT3 MRF+
The lower image shows a cT3 tumor in the low rectum below the
level of the peritoneal reflection.
There is involvement of the mesorectum on the anterior side
(green line), but not of the peritoneum.
cT4a MRF+
A combination of involvement of the peritoneum and the mesorectal fascia is also possible, like in this case.
This is a tumor in the upper rectum with involvement of the peritoneum on the anterior side and involvement of the mesorectal fascia on the posterior side.
T4b – Invasion of surrounding organs or structures
T4b tumors invade other organs or structures that are situated outside the mesorectum.
The beforementioned 2021 consensus panel proposed that T4b stage should include invasion of the structures as mentioned in the table (reference).
Though this has been a topic of debate, the consensus panel proposed that T4b invasion should also include invasion of any fat that is situated in another anatomical compartment outside the mesorectum (i.e. beyond the MRF), such as the obturator, parailiac or ischiorectal space.
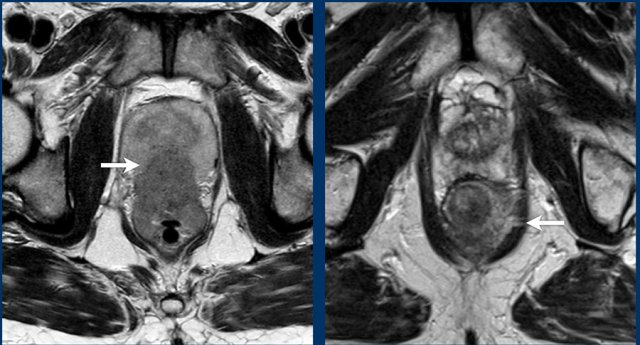
Examples of T4b disease with respective invasion of the prostate (left) and invasion of the levator ani (right).
Note
Invasion of striated muscles is considered
T4b disease, which includes invasion of the external anal sphincter,
puborectalis and levator ani muscles.
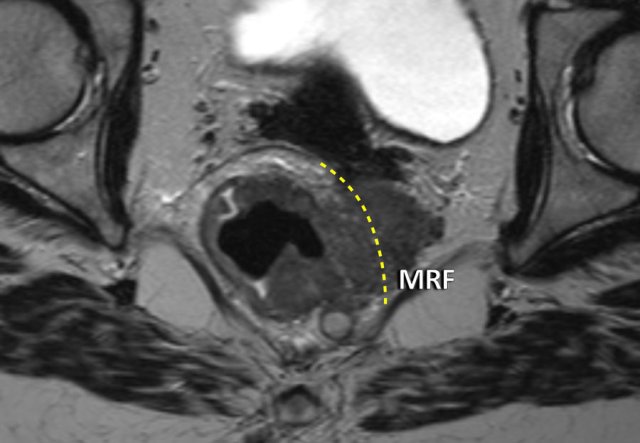
This is an example of a cT4b tumor growing beyond the mesorectal compartment into the fat of the obturator space.
Recognizing T4b invasion
Tumor invasion is defined a continuation of tumoral signal extending into an adjacent organ or structure, which is often accompanied by as loss of the intervening fat plane .
Scroll through the axial images and see how the intermediate signal intensity of the tumor is seen to extend into the posterior wall of the vagina (arrows).
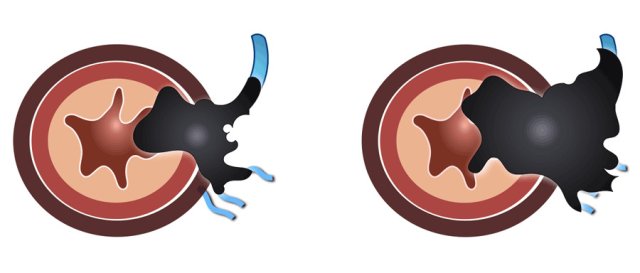
EMVI -Extramural vascular invasion
Extramural vascular invasion is a risk factor for recurrent disease, metastases and impaired overall survival.
EMVI is suspected if we see tumor-signal extending into a vascular structure in close proximity to the tumor, when the vessel is expanded by tumor, or if the tumor infiltrates the vessel borders (illustration).
Example of an EMVI+ tumor with tumor signal extending into an adjacent vessel
structure, expanding and disrupting the vessel contour.
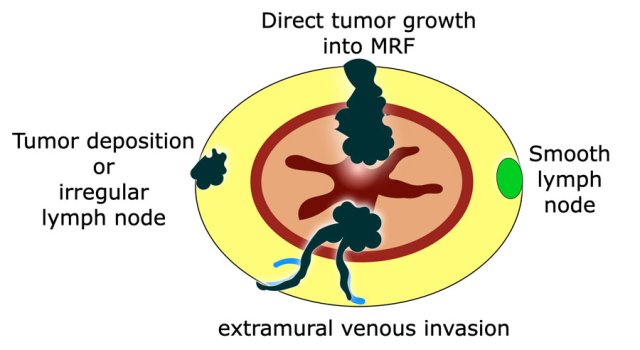
Pitfall: How to report MRF involvement by nodes, tumor deposits or EMVI?
In current guidelines it is not clearly described how to report MRF involvement by tumor-bearing structures other than the primary tumor.
The beforementioned multidisciplinary panel of experts proposed in 2021 that the MRF should be reported as involved in case of a ≤1 mm margin from either the primary tumor, extramural venous invasion or from irregularly enlarged tumor deposits or from irregular lymph nodes.
Potentially malignant, enlarged lymph nodes with a smooth margin and an apparently intact capsule contacting the MRF have a very low risk to result in margin involvement at histopathology and should therefore not be considered as MRF+ to avoid overtreatment.
Anal sphincter and pelvic floor involvement
The anal sphincter is
comprised of three layers:
- Internal sphincter: continuance of the smooth muscle of the rectum
- Intersphincteric space: between the internal and external sphincter
- External
sphincter: voluntary striated (skeletal) muscle. Cranially, the external sphincter is continuous with the puborectal and levator
ani muscles.
Together with the iliococcygeus and pubococcygeus muscles, the puborectalis and levator ani muscles form the “pelvic floor ”
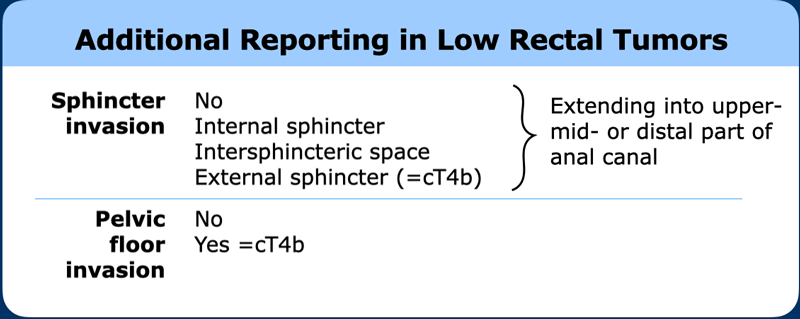
In
low rectal cancers, the MRI report should describe the relationship of
the tumor to the anal sphincter and pelvic floor to guide surgical and
radiotherapy planning.
This should include a description of which layers of the
anal sphincter and/or pelvic floor are involved, and whether invasion extends
into the upper, middle or lower thirds of the anal canal.
Pitfall anal sphinter and TNM
The TNM staging system does not clearly define how invasion of different layers of the anal sphincter and pelvic floor should be taken into account.
In the 2021 expert consensus meeting it was proposed to:
- Define the cT-stage primarily based on the extent of tumor invasion at the level of the rectum.
- Exclude involvement of the internal sphincter and intersphincteric plane for T-stage categorization
- Categorize involvement of the external sphincter, puborectalis or levator ani muscles (= skeletal muscle invasion) as cT4b disease.
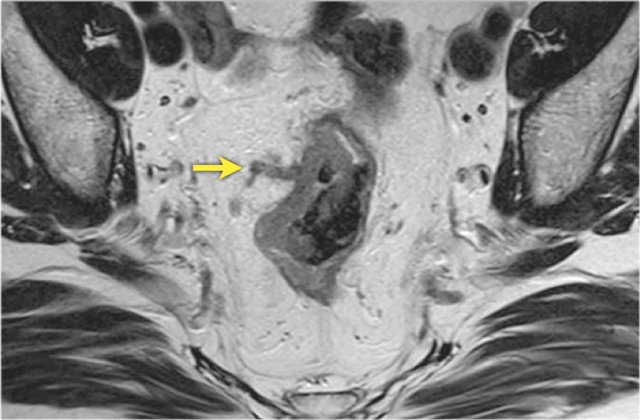
A shows an example of a cT1-2 low rectal tumor that invades the internal sphincter on the left side (arrow). Remember that the invasion of the internal sphincter does not impact the cT-stage, but should be mentioned explicitly in the MRI report to help guide surgical planning.
B is an example of a low rectal tumor that invades the internal and external sphincter on the right side (arrowheads).
There is also involvement of the puborectalis and levator ani muscles.
Remember that invasion of the external sphincter, puborectalis and levator equals skeletal muscle invasion and should be staged as cT4b disease.
Note the normal appearance of the external sphincter and pelvic floor muscles on the left side (green arrow).
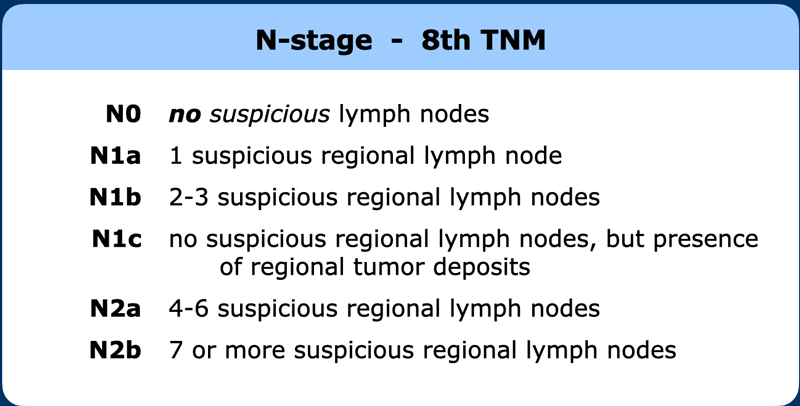
N-stage
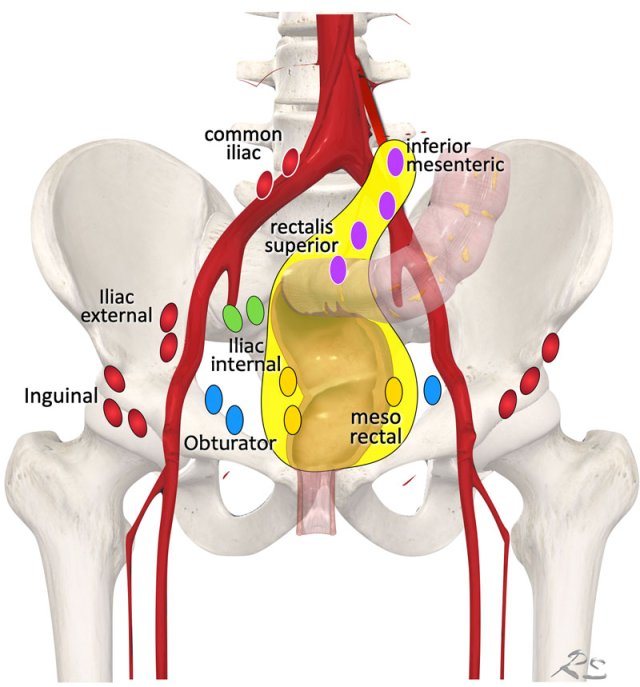 Regional lymph node drainage. The lymph nodes in red are all non, regional (M-stage) nodes. In TME only the mesorectal nodes and in high rectal tumors also the rectalis superior and inferior mesenteric nodes are excised.
Regional lymph node drainage. The lymph nodes in red are all non, regional (M-stage) nodes. In TME only the mesorectal nodes and in high rectal tumors also the rectalis superior and inferior mesenteric nodes are excised.
Lymph Node Map
The terminology used to describe the various lymph node stations in rectal cancer can be a source of confusion.
Regional lymph nodes
include all nodes that are part of the N-stage:
- Mesorectal lymph nodes (yellow).
- Nodes in the mesocolon of the distal sigmoid, along the “presacral” inferior mesenteric and rectalis superior blood vessels (purple)
- Nodes in the obturator spaces (blue)
- Nodes in the internal iliac spaces (green)
Notice that in standard TME only the mesorectal nodes are excised and in high rectal tumors also the rectalis superior and inferior mesenteric nodes (yellow area).
This means that other regional lymph nodes, which are located lateral to the mesorectum like the obturator and internal iliac nodes are not routinely excised.
Non-regional lymph nodes (all in red)
include all nodes that – when involved – are considered distant nodal metastases and are therefore part of the M-stage:
- External iliac nodes
- Common iliac nodes
- Inguinal nodes
Note
As an exception to this rule the AJCC TNM (8th ed.) considers inguinal nodes as regional nodes in case of low rectal tumors extending into the distal anal canal, below the level of the dentate line.
This MR-image shows the mesorectal, internal iliac, obturator and external iliac lymph node compartments.
Remember that external iliac nodes are non-regional and if positive they are regarded as metastatic disease.
The obturator and internal iliac space are divided by the lateral border of the main trunk of the internal iliac vessels.
The posterior border of the external iliac compartment is defined by the posterior border of the external iliac vessels [ref].
In the purple area the superior rectal and inferior mesenteric nodes are located.
These nodes are sometimes referred to as “high mesorectal nodes” and are part of the regional N-stage nodes.
The level of the highest suspicious node in this region should be mentioned in the report, as this will impact the chosen radiotherapy field.
Mesorectal lymph nodes
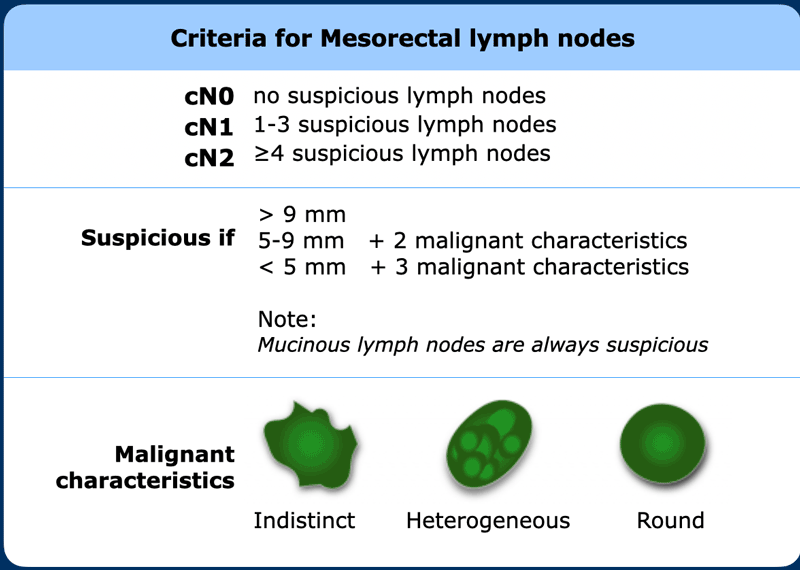
The N-stage in rectal cancer is only based on the number of suspicious regional lymph nodes.
Suspicious non-regional lymph nodes are considered metastatic disease.
MRI like other imaging modalities has a relatively low diagnostic performance to stage lymph nodes. Whether or not N-stage as determined by imaging should be taken into account for treatment stratification has been the topic of debate, though most current guidelines still consider positive N-stage on imaging a high risk sign, warranting neoadjuvant treatment.
Best
results are obtained when applying a combination of nodal size and morphology
to characterize mesorectal lymph nodes.
See published guidelines by ESGAR and in the Dutch national guidelines [ref].
Nodes ≥ 9 mm and nodes with mucinous signal characteristics
are always regarded as suspicious.
Smaller lymph nodes require additional morphologically
suspicious features (round shape, indistinct border, heterogeneous signal) in
order to be considered as cN+ as detailed in the Table.
Note
It is important to mention the level of the most proximal suspicious lymph node
especially if there are N+ nodes present high in the mesorectum or in the distal
mesosigmoid, along the superior rectal or inferior mesenteric vessels, as these
nodes can impact the radiotherapy field. The same size + morphology criteria
apply to stage these “high mesorectal” nodes.
Lateral lymph nodes
Lateral lymph nodes are sometimes referred to as extramesorectal lymph nodes.
These are lymph nodes that are located lateral to the mesorectum and are not routinely removed in standard TME resection.
They need
to be reviewed carefully and mentioned separately in the report.
Especially tumors
located below the peritoneal reflection have a tendency to spread to the
internal iliac and obturator compartments.
If pathologic
nodes in these compartments are not additionally treated by lateral nodal
dissection or radiotherapy, they are associated with a higher risk for
recurrence.
In
2019, the lateral nodal study consortium proposed a size cut-off of ≥7 mm short axis diameter to stage internal iliac and obturator nodes on MRI and
also showed that – unlike in mesorectal nodes – morphologic features should not
be taken into account (ref).
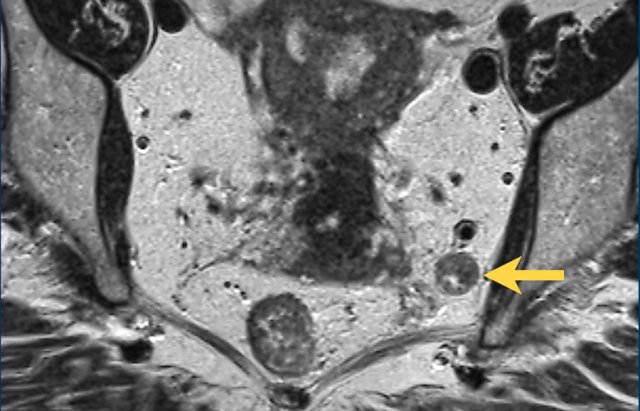
Example of a pathologic lymph node measuring 9 mm in the left obturator space (arrow).
This node needs to be irradiated or resected separately to avoid recurrence.
This axial T2W-image is of a patient who was treated with a TME.
There is a local recurrence of rectal cancer due to an untreated positive lateral lymph node
Although not routinely used by all for radiological staging, the 8th edition of the TNM further divides the N-stage (table).
Pitfall: nodes versus tumor deposits
Pathologic lymph nodes and tumor deposits can look very similar on imaging and there are no widely adopted criteria to discriminate the two. Some define tumor deposits as more irregular nodules that are often situated within or along vessels, while pathologic nodes still have a familiar round or oval shape and capsule typical of lymph nodes (ref).
These definitions, however, remain to be validated at large scale. Until then, the 2021 consensus panel advised to group nodes and deposits together in the cN-stage.
A prose description of the size and morphology of the suspicious lesions should be included in the report (ref).
Image
This sagittal T2W-image shows a low rectal cancer with multiple irregular nodular lesions in the mesorectal fat on the posterior side. Though one could argue whether these lesions represent tumor deposits or pathologic lymph nodes, they are all considered as part of the N-stage, which was cN2 in this patient.
Combined with advanced T-stage (T3c MRF+) this patient was classified as locally advanced and received neoadjuvant chemoradiation for tumor and nodal down-staging.
M-stage
The M-stage in rectal cancer is based on the presence of suspicious non-regional lymph node metastases and other distant metastases.
Note that non-regional lymph nodes are together considered as one “organ”.
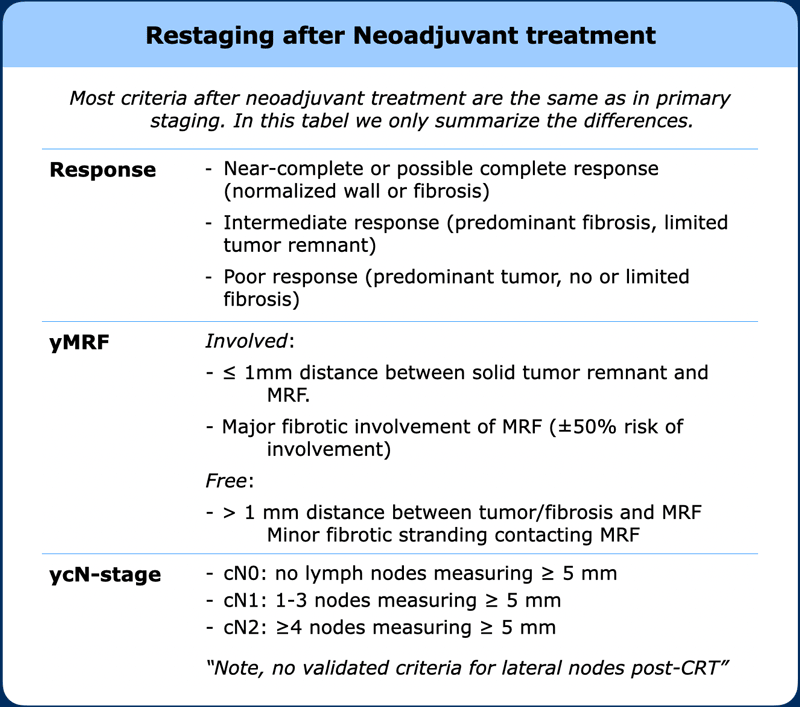
Restaging after neoadjuvant treatment
Checklist
A restaging report basically uses the same descriptors as for primary staging.
In addition to yTNM-staging, it is important to give an overall estimation of the degree of response and classify patients as poor, good or potential complete responders to inform further clinical decision making.
In
most cases, a restaging report mainly serves as an up-to-date roadmap for the
surgeon.
In some cases restaging is
also used to select potential candidates for organ-preservation.
In the table the main items and criteria that are specific to the restaging setting are summarized.
Response assessment
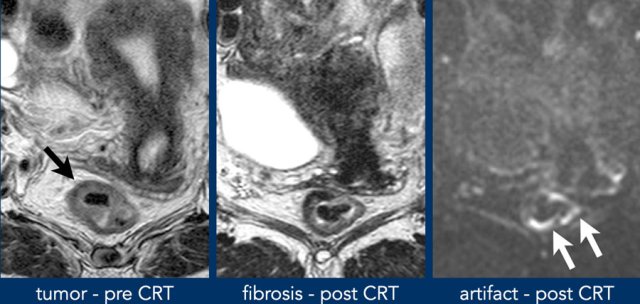
After
chemo-radiotherapy, rectal tumors typically decrease in size and undergo a
fibrotic transformation which can be observed as a marked decrease in signal
intensity of the tumor bed on T2-weighted images.
In a small minority of cases (<5%) the tumor completely disappears and an apparently normalized rectal wall re-appears on MRI after CRT.
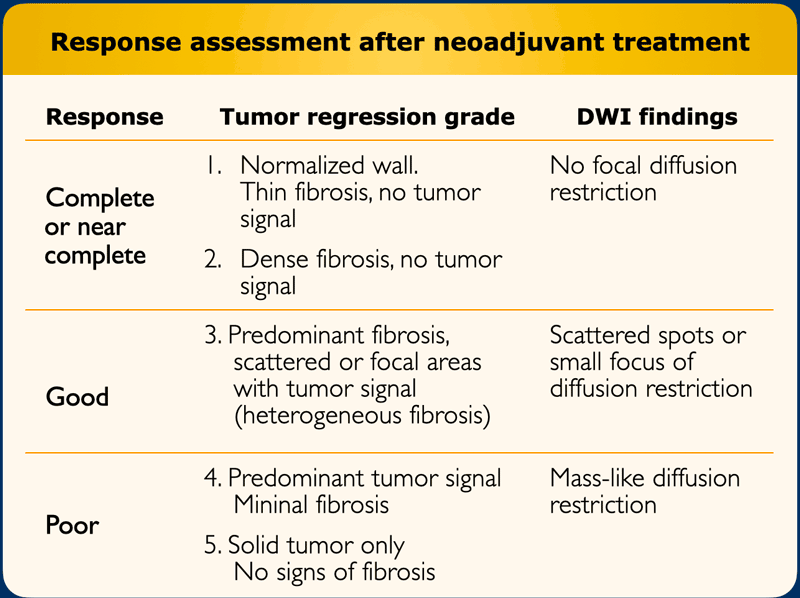
A restaging MRI report should start with a general description of the degree of response.
The
response can be classified into:
- Near-complete or possible complete response when there is a normalized rectal wall or only fibrosis
- Good response with predominant fibrosis but suspicion of small tumor remnant within the fibrosis on T2-weighted MRI or DWI
- Poor response with predominantly solid tumor remnant
Tumor regression grade (TRG)
MRI has known difficulties in differentiating between
fibrosis still containing vital tumor cells and mere fibrosis.
Nevertheless there are certain patterns that can help
estimate the risk for significant viable tumor within the fibrosis.
The MR tumor regression grade (mrTRG) is an imaging adaptation of similar TRG systems used at histopathology and can be used to grade the degree of fibrotic transformation on T2-weighted MRI using a 5-point scale (table)
Diffusion-weighted imaging
DWI
highlights tissue with a high cellular density in which the extracellular
movement of water is “restricted”.
DWI has been shown to be a useful adjunct to T2-weighted MRI
to diagnose the presence of viable residual tumor within the fibrotically
changed tumor bed after CRT [reference].
In case of residual tumor, a high signal can typically be
observed at the inner margin of the fibrosis on high b-value diffusion-weighted
images, with a corresponding low signal on the ADC map.
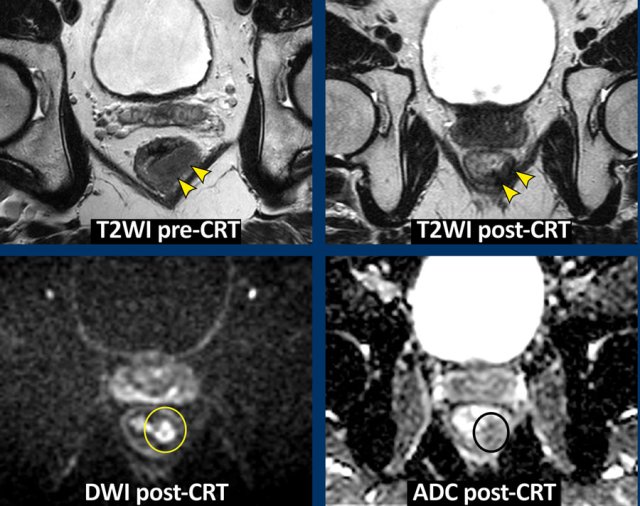
Images
The
images show the primary staging and restaging T2-weighted images after
chemoradiotherapy with predominant fibrosis with minor signal heterogeneity
(TRG 3).
The corresponding restaging DWI shows a focal area of high
signal at the inner margin of the fibrosis with corresponding low signal on the
ADC map, indicating restricted diffusion.
This
was confirmed to be a small tumor remnant (ypT2) at histopathology.
Pitfall: staging in case of fibrosis
Unfortunately
the overall accuracy of MRI to assess yT-stage, yMRF, yEMVI and sphincter
invasion after CRT is poorer than in the primary staging setting due to the
difficulties of MRI to assess the presence and extent of vital tumour within
the fibrotically changed tumor bed.
Assessment of MRF involvement after CRT
When a fat plane re-appears between the tumor bed and the MRF after CRT, the risk of persistent MRF
involvement is very small.
When
there is still diffuse infiltration of the MRF by intermediate tumor signal
after CRT, the risk for tumor invasion at histopathology is high (around
90%).
The
most difficult cases are those with diffuse fibrotic infiltration of the MRF.
In these cases, the risk for MRF positivity at histopathology
is around 50% [reference].
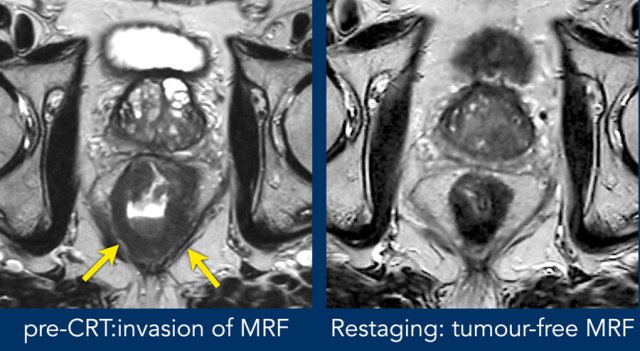
Images
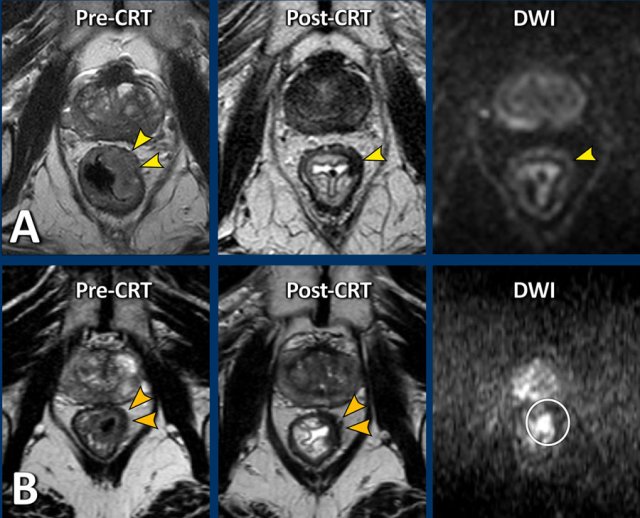
Pre-CTR there is extensive invasion of the MRF from 4-8 o’clock (arrows). After CRT the tumor has undergone a fibrotic transformation and has retracted from the MRF. A fatplane has appeared with only some minor fibrotic stranding towards the MRF.
These are signs indicative of a tumor-free MRF at restaging (yMRF-)
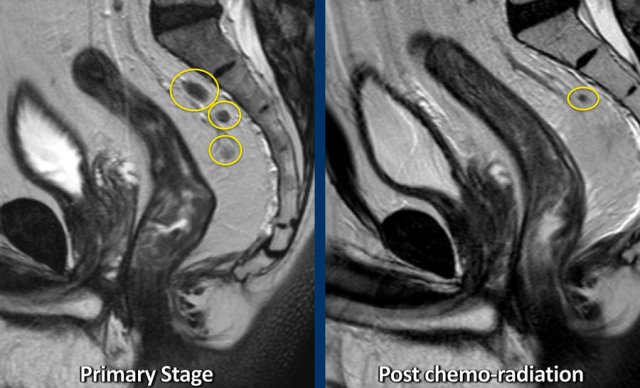 Example of a patient with several irregularly enlarged cN+ nodes at primary staging. After chemoradiotherapy, most nodes have disappeared and only a small node of < 5 mm remains, indicative of a ycN0 stage.
Example of a patient with several irregularly enlarged cN+ nodes at primary staging. After chemoradiotherapy, most nodes have disappeared and only a small node of < 5 mm remains, indicative of a ycN0 stage.
yN-stage
The diagnostic performance of MRI to restage lymph nodes after CRT is better than for the primary staging of nodes.
After CRT, the majority of nodes decrease in size or completely disappear on MRI. Nodes that remain clearly visible after CRT are still at risk.
Although the optimal size cut-off remains a topic of debate, a cut-off of ≥5 mm (short axis diameter) has been proposed to diagnose yN+ nodes after CRT [ref].
For the lateral nodes, the lateral nodal study consortium have proposed a cut-off of >4 mm (internal iliac) and >6 mm (obturator), but these criteria are to date considered preliminary and remain to be validated [ref].
Lymph nodes like other lymphoid tissues including the spleen have a dense cellular structure resulting in restricted diffusion and a high signal on DWI.
As a result, DWI can be helpful in detecting lymph nodes but is less suitable for lymph node characterization as both benign and metastatic lymph nodes will show high signal.
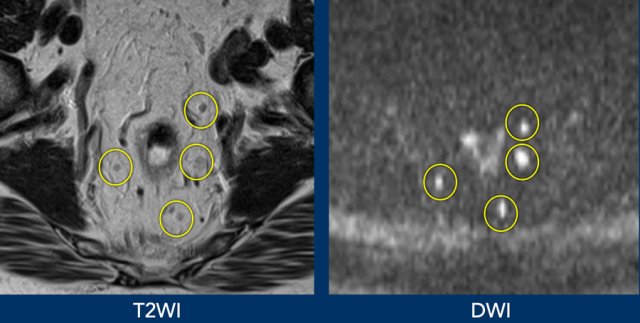
Images
Better visualisation of lymph nodes on DWI compared to corresponding T2WI.
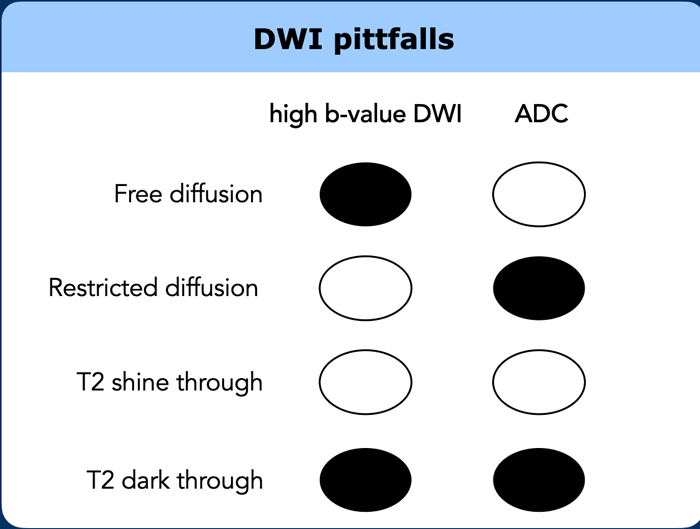
DWI pitfalls
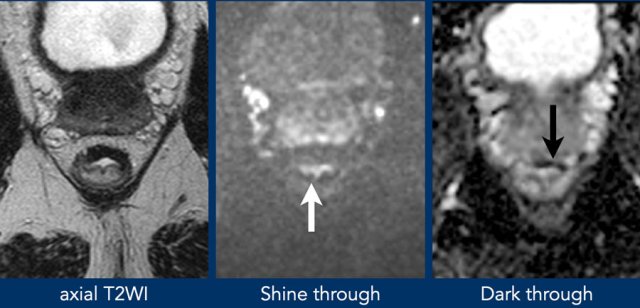
T2 shine through
Diffusion-weighted
images are inherently T2-weighted.
T2 shine through refers to the presence of high signal on
DWI that is not caused by restricted diffusion, but by long T2-relaxation time
(e.g., in fluids).
In rectal DWI this may occur in case of small amounts of
fluid in the rectal lumen, which may mimic tumor in the adjacent rectal wall.
To differentiate between this luminal T2 shine-through and tumor one should refer
to the ADC map where luminal fluids will show a high signal.
T2 dark through
Also called T2 black out, refers to the
markedly low signal observed on the ADC map in areas of dense fibrosis without
vital tumor.
This occurs in tissues with a very short T2-relaxation time (such
as collagen-rich fibrosis, calcified lesions and cortical bone) and will result
in a completely hypointense signal on the ADC map, but also on other series
including the DWI, T2-weighted and T1-weighted sequences.
T2 dark through
should not be mistaken for restricted diffusion suspicious for tumor.
Example of shine through of high T2 signal on DWI from fluid in the rectal lumen.
The ADC map shows an example of T2 dark through with distintly low signal in the fibrotically changed rectal wall.
There is no corresponding high signal in the wall on DWI, indicating that there is no actual diffusion restriction.
Susceptibility artefacts
Abdominal DWI scans are often acquired using echo planar imaging (EPI), which allows fast image acquisition thereby minimizing the risk of motion artefacts.
The main downside of EPI-DWI is that it is highly prone to susceptibility effects, i.e. distortions or artificial pile up of MR signal caused by local field inhomogeneities, especially at higher field strengths.
In rectal DWI, these susceptibility effects mainly occur at the interface between soft tissue in the rectal wall or tumor and gas in the lumen.
While large artefacts will be easy to recognize as artefacts, more subtle ones projecting over the rectal wall may be erroneously interpreted as tumor signal.
Susceptibility artefacts in rectal DWI may be avoided by reducing the amount of gas in the rectal lumen or by using alternative methods of DWI acquisition, such as spin echo techniques, that are less prone to these susceptibility effects.
Images
This is a patient with tumor in the right anterolateral rectal wall. Post CRT there is fibrosis visible at the site of the former tumor bed from 9-12 o’clock.
The high signal on DWI is located on the contralateral side, outside the tumor bed and corresponds to susceptibility artefacts caused by gas in the rectal lumen. These artefacts should not be mistaken for high signal suspicious of tumor.
MR protocol
Hardware
MRI of rectal cancer may be performed at either 1.5T or 3.0T,
using phased array external surface coils. Use
of an endorectal coil is not routinely recommended.
Patient preparation
Patient preparation is not mandatory.
Use of spasmolytics may be considered to reduce bowel movement artefacts
(particularly in upper rectal tumors that are more prone to these
artefacts).
Use
of endorectal filling is not routinely recommended since distension of the
rectum and consequent compression of perirectal tissues may interfere with
correct interpretation of the distance between the tumor and mesorectal fascia
.
Preparatory
steps to reduce the amount of gas in the rectal lumen may be helpful to avoid
gas-induced susceptibility artifacts on DWI-sequences, although this is mainly
an issue in the restaging setting where DWI plays a more important role.
This
can be achieved for example by giving patients a preparatory micro-enema
or a small volume of rectal filling (up to 60 ml).
Sagittal series
The sagittal series is used to localize the tumor and to plan the
axial and coronal series. The cranial border of the field of view (FOV) should
be at the level of the sacral promontory and the caudal border below the anal
canal.
Axial series
The axial (or oblique-axial) view should be angled perpendicular
to the tumor axis to allow proper assessment of the extension of the tumor
beyond the rectal wall and the distance between the tumor and MRF.
Coronal series
The coronal
sequence should be angled parallel to the tumor axis, which is perpendicular to the axial series.
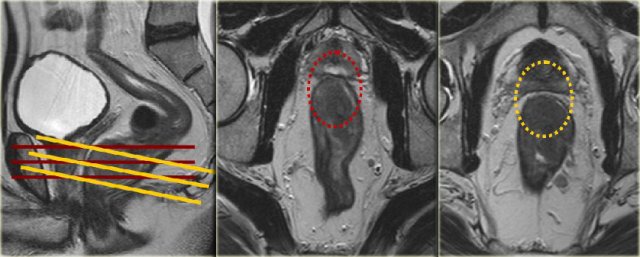
In distal tumors near or involving the anal canal, the coronal sequences should be planned parallel to the anal canal or an additional coronal plane parallel to the anal canal should be added to the protocol to properly assess whether and to what extent the tumor is invading the anal sphincter (right figure).
Example showing
impact of sequence angulation:
In the middle
image the axial view is angled in true axial plane, which is not perpendicular to the tumor axis of this low rectal tumor.
This resulted in the false impression that the MRF was involved on the anterior
side (red circle).
In the right image, the axial view is correctly planned
perpendicular to the tumor axis and it was clear that the MRF was not involved
(yellow circle).
T2WI
A rectal
MRI protocol should routinely include high resolution 2D T2-weighted sequences
in multiple planes with a slice thickness of ≤3 mm. Although recent technical advances have
improved the quality of 3D T2-weighted sequences, they are not yet commonly
used as a replacement for 2D T2-weighted sequences. The required in plane
resolution is less well documented in guidelines, though a resolution of 0.6 x
0.6 mm or less is generally recommended [ref].
DWI
It is recommended to routinely include a diffusion-weighted imaging (DWI) sequence. Diffusion weighted imaging can be useful for tumor and lymph node detection in primary staging and are particularly useful for the restaging of tumors after neoadjuvant treatment.
The DWI protocol should include at least one high b-value of ≥ 800 s/mm2.
Apparent diffusion coefficient maps should be calculated from the DWI series to be studied visually alongside the DW images (see also section on DWI pitfalls).
Images
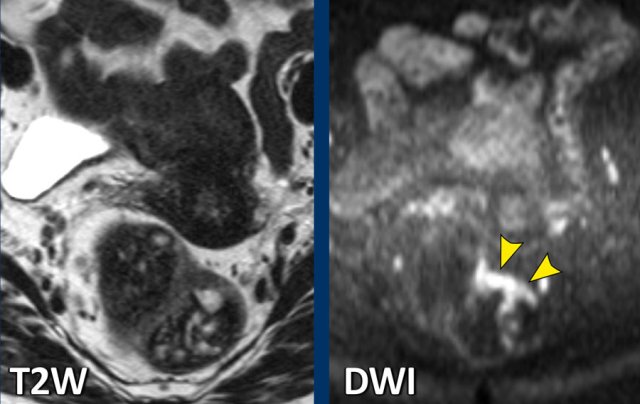
Example of a patient with a lot of faeces in the rectum. The tumor itself is barely recognizable on the T2-weighted MRI, but easy to locate on DWI.
Here are two more examples of patients with very similar semicircular tumors pre- and post-CRT.
On the T2W-images post-CRT both patients show some fibrotic wall thickening in the radiated area, but no obvious solid tumor remnant (yellow arrowheads).
On DWI, patient A shows no mass-like diffusion restriction. There is only some shine through of fluid signal in the lumen.
In patient B there is focal restricted diffusion at the site of the fibrosis.
Patient A was confirmed to be a complete responder at endoscopy and went into a wait-and-see program.
Patient B underwent resection which confirmed a ypT2 tumor remnant.
Other sequences
T1-weighted MRI: non-enhanced T1-weighted sequences may be useful to help characterize coincidental findings (e.g. bone lesions, ovarian cysts) but are not mandatory for staging . T1-weighted sequences with an extended field of view can also be used to cover all relevant lymph node stations within a relatively short acquisition time.
Intravenous contrast: steady-state gadolinium enhanced imaging does not improve diagnostic accuracy for clinical staging and is not routinely recommended. Dynamic contrast-enhanced MRI is not routinely recommended for clinical staging.
Fatsuppression: fatsuppressed sequences are not required for staging. T2-weighted fatsuppressed images may be of added benefit for patients with concommittant perianal fistulas or abscesses.
Surgery
Total mesorectal excision - TME
The
current standard surgical procedure for rectal cancer is the total mesorectal
excision (TME).
TME is an umbrella term that is used to describe different
surgical techniques (LAR, APR) that include a total resection of the mesorectum
along the mesorectal fascia.
Low anterior resection - LAR
With a low anterior resection, the anal canal is left in situ
and an anastomosis is made between the rectum and sigmoid colon. LAR is
therefore typically applied in middle and upper rectal tumors where there is
sufficient margin between the lower tumor border and the anal canal to make an
anastomosis, most commonly using a “side-to-end” anastomosis
Abdominoperineal resection - APR
With an abdominoperineal resection, the rectum and anal canal are
resected en bloc and the patient receives a permanent colostomy. APR is
indicated for low rectal tumors with a close margin or involvement of the anal
canal.
Perineal repair techniques after APR include primary surgical closure, filling of the pelvis using omentoplasty, or plastic reconstructive techniques including vertical or oblique rectus abdominis (VRAM/ORAM) myocutaneous flaps.
Intersphincteric APR
The intersphincteric is a variation to the standard APR where the external sphincter is spared.
Extralevator APR
This is a more extensive procedure
which includes the levator ani muscles and is indicated for tumors that
invade the pelvic floor (levator).
Local Resection
This is an
umbrella term for various minimally invasive techniques to excise rectal tumors
endoscopically, through the anus. Endoscopic mucosal resection (EMR) and
endoscopic submucosal resection (ESD) are superficial excision techniques used
for non-cancerous polyps and T1a and T1b tumors.
Transanal minimally invasive
surgery (TAMIS) or transanal endoscopic microsurgery (TEM; a very similar but
older technique) is a full thickness endoscopic resection of all layers of the
bowel wall that can be applied for T1 (and some small T2) tumors.
Organ-preservation
There
is a growing tendency to consider minimally invasive or non-surgical treatment
alternatives in tumors that show a complete or near-complete response after
neoadjuvant treatment.
These alternatives include the “watch-and-wait” strategy, where patients with a clinical complete response after neoadjuvant treatment are deferred from surgery and closely monitored, and local excision or local radiotherapy techniques for patients with small tumor remnants.
These developments have urged the need for a more accurate radiological assessment after neoadjuvant treatment and MRI – combined with endoscopy and clinical examination – plays an important role in the selection and monitoring of these patients.
Video examples of Staging
Low Rectal cancer
In this case we demonstrate how to stage a low rectal cancer.
You can scroll through the images and then go to the video in wich we will explain the staging.
In this video we demonstrate how to stage a low rectal cancer.
High Rectal cancer
In this case we demonstrate how to stage a high rectal cancer.
You can scroll through the images and then go to the video in wich we will explain the staging.
In this video we demonstrate how to stage a cancer in the upper or high rectum.
With special attention for the anterior peritoneal reflexion, mesorectal fascia and extramural vascular invasion.
If you want to scroll through the images yourself, you may first wanna look at the scroll images.
Charity
All the profits of the Radiology Assistant go to Medical Action Myanmar which is run by Dr. Nini Tun and Dr. Frank Smithuis sr, who is a professor at Oxford university and happens to be the brother of Robin Smithuis.
Click here to watch the video of Medical Action Myanmar and if you like the Radiology Assistant, please support Medical Action Myanmar with a small gift.