Pancreatic cystic Lesions
Diagnosis and management
Marc Engelbrecht, Jennifer Bradshaw and Robin Smithuis
Radiology department of the Academical Medical Centre, Amsterdam and the Alrijne hospital in Leiderdorp, the Netherlands
Publicationdate
Cystic pancreatic lesions are increasingly identified due to the widespread use of CT and MRI.
Certain pancreatic cysts represent premalignant lesions and may transform into mucin-producing adenocarcinoma.
Although the overall risk of malignancy is very low, the presence of these pancreatic cysts is associated with a large degree of anxiety and further medical investigation due to concerns about malignancy.
In larger cystic lesions it is usually possible to differentiate between benign serous cystadenomas and premalignant mucinous cystic neoplasms and intraductal pancreatic mucinous neoplasms, but in small lesions characterization is often not possible.
This means that many pancreatic cysts remain undetermined and guidelines are needed for follow up and management
Introduction
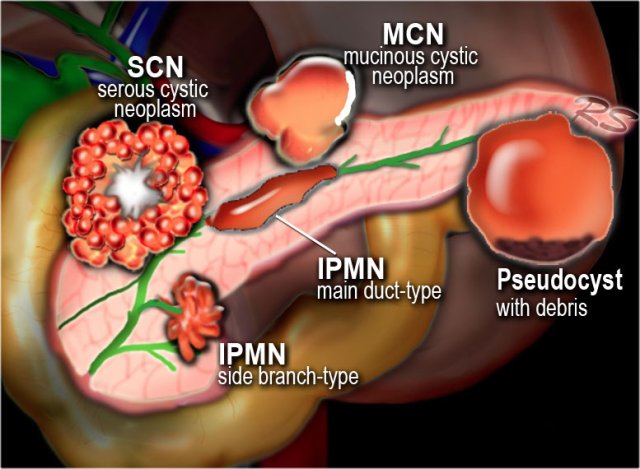
Classification
Pancreatic cysts can be categorized into the following groups:
- Pseudocysts
-
Common cystic neoplasms:
- IPMN - intraductal papillary mucinous neoplasm
- SCN - Serous cystic neoplasm
- MCN - Mucinous cystic neoplasm
-
Uncommon cystic neoplasms:
- SPEN (solid pseudopapillary epithelial neoplasm)
-
Tumors with cystic degeneration:
adenocarcinoma - neuroendocrine tumor
Systematic Approach
When a cystic pancreatic lesion is detected, the first step is to decide whether the lesion is most likely a pseudocyst or a cystic neoplasm.
This scheme is a simplified roadmap for the differentiation of pancreatic cysts.
- Pseudocyst - Think pseudocyst when there is a history of chronic or acute pancreatitis, alcohol abuse, stone disease or abdominal trauma. Findings suggestive of chronic pancreatitis may be parenchymal or ductal calcifications and peripancreatic fat-infiltration.
If cyst amylase is lower than 250 u/ml a pseudocyst is excluded (3) - Cystic neoplasm- No history of pancreatitis or trauma, or when the cyst has internal septa, a solid component, central scar or wall calcification.
- Mucinous cystic neoplasm - This is usually a unilocular cyst filled with mucin sometimes with wall calcification, exclusively seen in predominantly 40 – 60 year old women. They are often located in the body or tail and are characterized by ovarian type stroma in the pathological evaluation.
- Serous cystic neoplasm - This is most often a microcystic lesion that contains serous fluid. SCN may have various appearances, with 45% microcystic (cysts < 2 cm), 32% macrocystic, 18% mixed microcystic and macrocystic and 5% solid (4).
Microcystic SCN can be hypervascular and appear solid on contrast enhanced CT and therefore easily confused with pancreatic adenocarcinoma. MRI will clearly show the microcystic nature of the lesion (5) (6). - Branch-duct IPMN - This tumor can look like a SCN, but has no scar or calcifications. MRCP or heavily weigted T2WI may show the connection to the pancreatic duct, which is highly specific, but in many cases we are not able to detect this communication.
The left CT-image is of a patient with a history of pancreatitis.
There are two unilocular or simple cysts.
Notice also the retroperitoneal fat-stranding on the right.
The most likely diagnosis is pseudocysts.
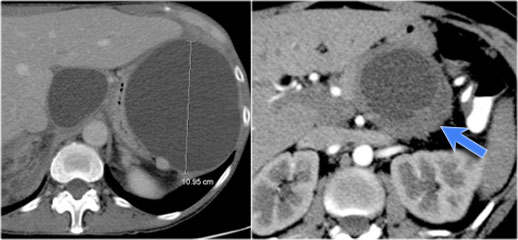
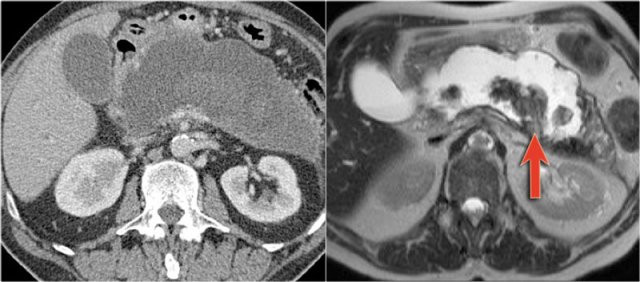
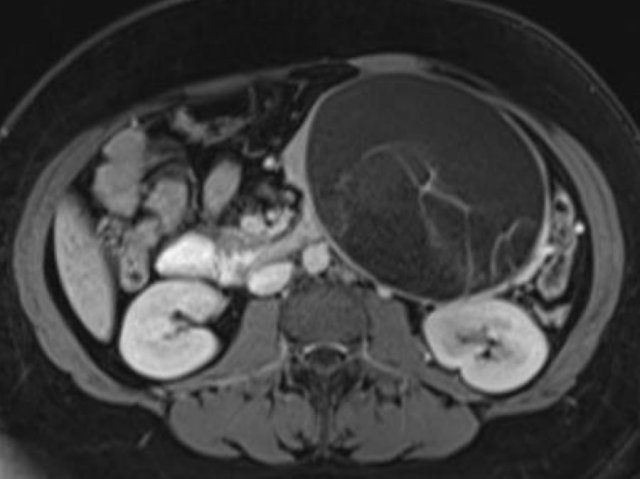
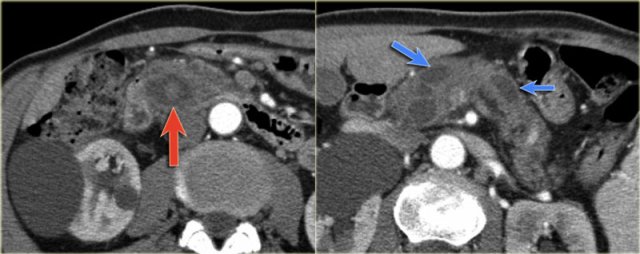
The CT on the right shows a cyst in the pancreatic tail in a 36 year old woman, which was found incidentally with US.
The cyst has a thick irregular rim and contains solid 'non-dependent' components.
The most likely diagnosis is a cystic neoplasm.
MRI versus CT
CT will depict most pancreatic lesions, but is sometimes unable to depict the cystic component.
MR with heavily weighted T2WI and MRCP will better demonstrate the cystic nature and the internal structure of the cyst and has the advantage of demonstrating the relationship of the cyst to the pancreatic duct as is seen in IPMN.
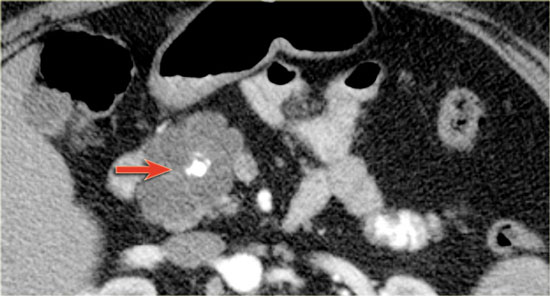
The images show a serous cystic neoplasm (SCN) on a CT.
MRI better shows the central scar.
There are cases when CT can be helpful, since it better depicts a central calcification in SCN or peripheral calcification in a mucinous cystic neoplasm (MCN).
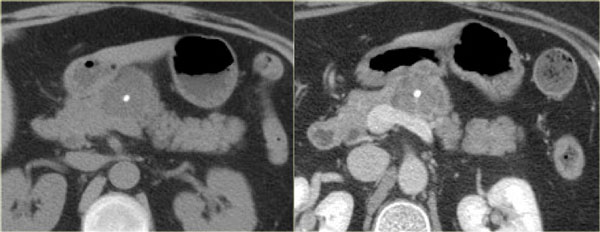
CT images of a mucinous cystic neoplasm with septations and peripheral calcifications.
MRI is usually of more diagnostic value than CT.
MRI can show the cystic nature of a pancreatic fluid collection and its internal structure.
The MRI shows a pancreatic fluid collection with dependent internal debris typical of walled off necrosis in necrotizing pancreatitis (7).
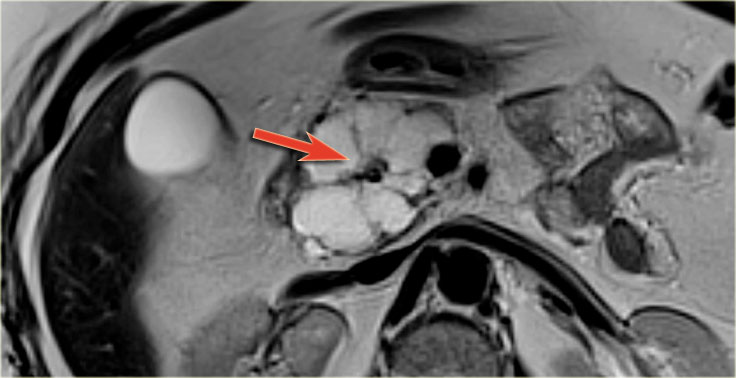
MRI shows a lesion, which consists of multiple small cysts.
This could be a serous cystic neoplasm or a branch-duct IPMN.
The connection of the cystic lesion to the pancreatic duct indicates that this is a branch-duct IPMN.
Pseudocyst
Key findings:
- Unilocular cyst without solid components, central scar or wall calcification.
- Collection of pancreatic enzymes, blood and necrotic tissue.
- Debris within a cystic lesion is a specific MR finding.
- History of pancreatitis or abdominal trauma.
- Cysts develop in 4-6 weeks - usually decrease in size over time - sometimes enlarge or become infected.
- Found in any part of the pancreas or anywhere within the abdomen and sometimes even in the chest.
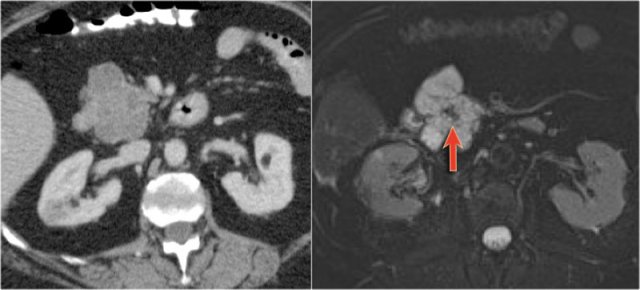
The CT demonstrates a large cyst in the upper abdomen in a patient who had an acute pancreatitis (Fig).
Notice that there is also some ascites and pleural fluid.
There is wall enhancement.
CT demonstrates two large cysts in a 45 year old woman, who had a trauma (fig).
Notice some fat stranding in the retroperitoneal space (arrow).
The imaging findings combined with the history make it very likely that these are traumatic pseudocysts.
Most pseudocyst occur in the peripancreatic region, but rarely they may extend to the mediastinum.
Scroll through the images.
This patient has a chronic pancreatitis.
Notice the calcifications in the pancreatic head (curved arrow).
There are multiple pseudocysts extending all the way to the mediastinum compressing the heart (red arrow).
Cystic Neoplasms - differential diagnosis
The diagnosis of a cystic neoplasm should be considered when there is no history of pancreatitis or trauma.
Morphological characteristics of a cystic neoplasm are:
- Thick irregular rim
- Septations,
- Solid components,
- Dilated pancreatic duct > 3mm and calcifications.
- Fluid aspirated from a neoplastic cyst will show low amylase level (3).
In the Table some discriminating features of cystic neoplasms.
In many cases however it is not possible to make a definitive diagnosis, because often the cyst will be too small.
However it is important to diagnose a serous cystic neoplasm, since this is the only tumor with no malignant potential. When there are symptoms it is due to increasing size.
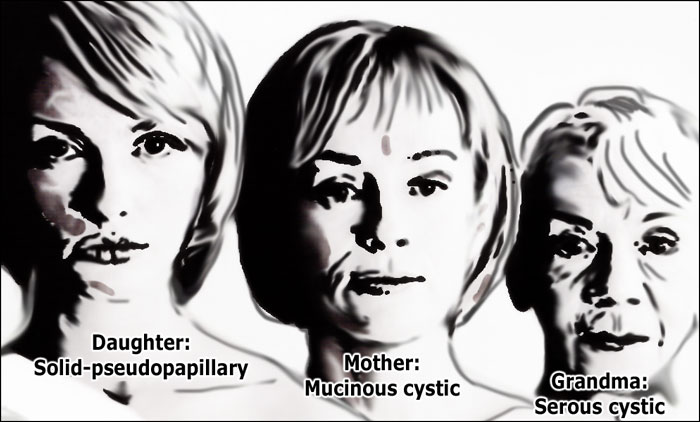
Age and gender
Mucinous cystic neoplasm
MCN is exclusively seen in middle-aged women with a mean age of 47 years (8) .
Only 12 cases reported in males up to date (9).
Serous cystic neoplasm
SCN is also most commonly seen in women (75%) with a median age of 58 years (4).
Solid pseudopapillary epithelial neoplasm
SPEN is seen exclusively in young women (88%), with a mean age of 29 years (10).
It is an uncommon solid tumor that may have cystic components.
Hence the following rule:
- Grandma - Serous cystic adenoma (SCN)
- Mother - Mucinous cystic neoplasm (MCN)
- Daughter - Solid pseudopapillary epithelial neoplasm (SPEN)
Serous cystic neoplasm
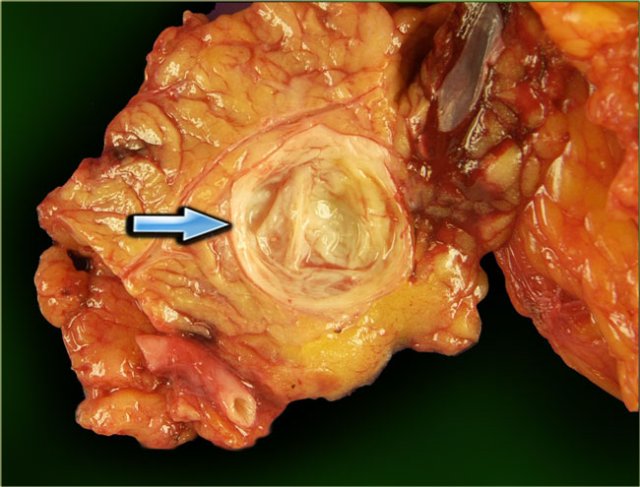
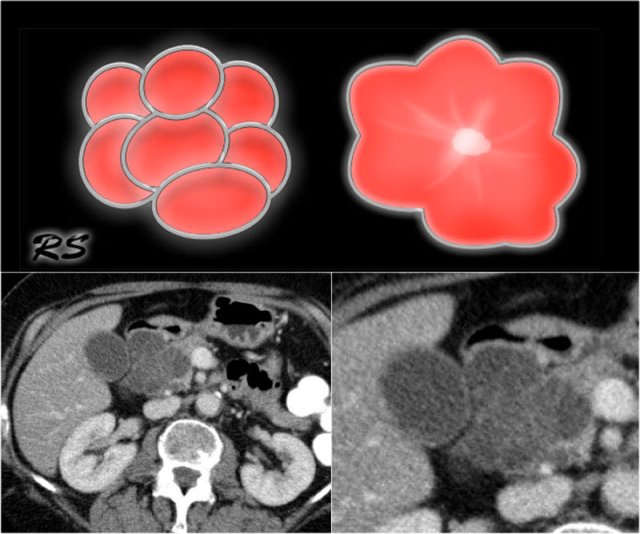 Figure 14. Serous cystic adenomas contain multiple small cysts resulting in a lobulated contour. Some have a central scar with calcifications.
Figure 14. Serous cystic adenomas contain multiple small cysts resulting in a lobulated contour. Some have a central scar with calcifications.
Key findings:
- Benign tumor, but large tumors have a tendency to increase in size and cause symptoms.
- Typically seen in 'Grandma'.
- SCN may have various appearances like microcystic (45%), macrocystic (32%), mixed microcystic and macrocystic (18%) and solid (5%) (4).
- Microcystic or honey-combed cyst with central scar (30%) and calcifications (18%).
- Macrocystic in 10% and difficult to differentiate from pseudocyst and mucinous cystic neoplasm.
- Lobulated surface.
- No communication between cysts and pancreatic duct.
- Hypervascular enhancement is sometimes seen and can be challenging to differentiate from cystic neuroendocrine tumor.
- Growth rate of tumors Growth rate of tumors > 4 cm: up to 20 mm/y.
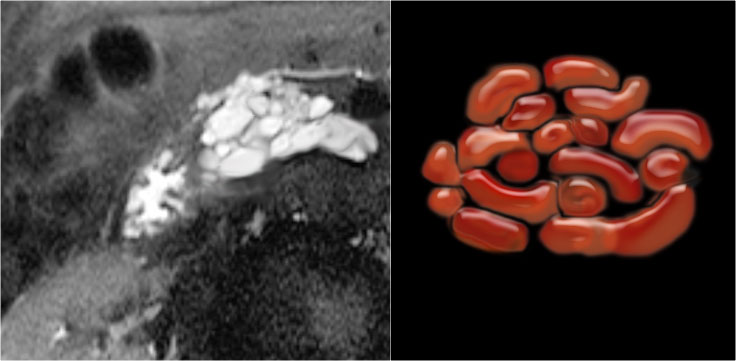
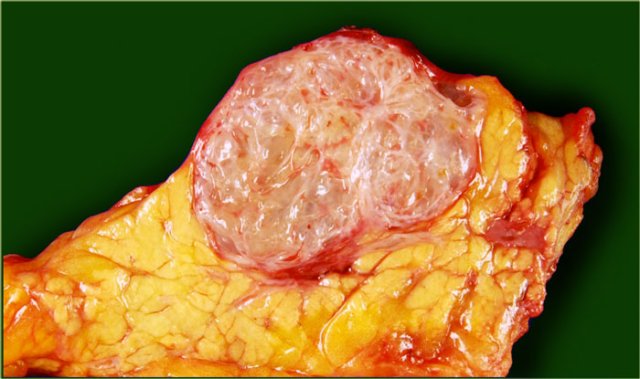 Serous cystic neoplasm with cmultiple small cysts. Courtesy of Dr Allen, HPB surgery, Memorial Sloan Kettering Cancer Center, NY
Serous cystic neoplasm with cmultiple small cysts. Courtesy of Dr Allen, HPB surgery, Memorial Sloan Kettering Cancer Center, NY
The pathology specimen shows multiple microcysts, which gives the tumor a lobulated appearance.
A macrocystic serous cystic neoplasm is rare and, although benign, can be similar in appearance to the potentially malignant macrocystic mucinous cystic neoplasm.
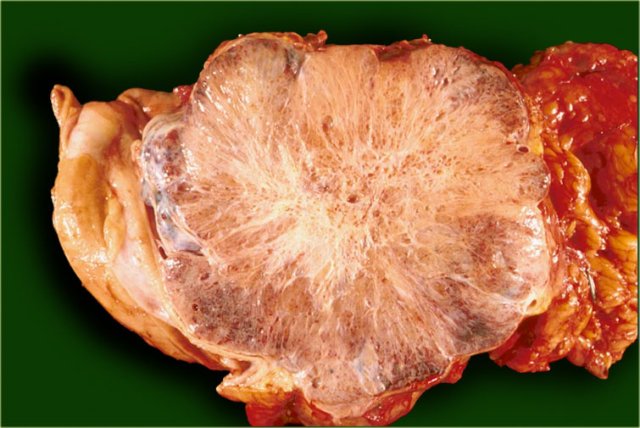 Serous cystic neoplasm with multiple small cysts. Courtesy of Dr Klimstra, pathology of the Memorial Sloan Kettering Cancer Center, NY,
Serous cystic neoplasm with multiple small cysts. Courtesy of Dr Klimstra, pathology of the Memorial Sloan Kettering Cancer Center, NY,
A characteristic feature of a serous cystic neoplasm is a central scar, sometimes with calcifications.
Sometimes the microcystic component of this tumor is difficult to identify on CT.
MR will better identify the internal architecture.
MRI is also useful in determining if the cysts communicate with the pancreatic duct or not to differentiate this lesion from a branch-duct IPMN (see below).
The pathology specimen shows a cystic tumor with multiple small cysts and a central scar.
There are no calcifications.
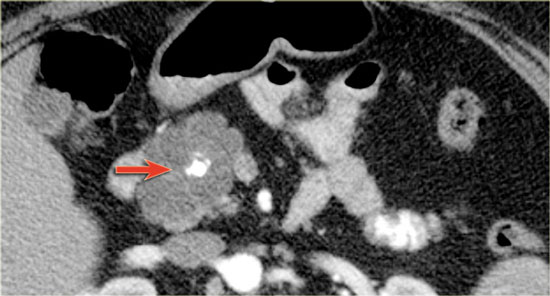
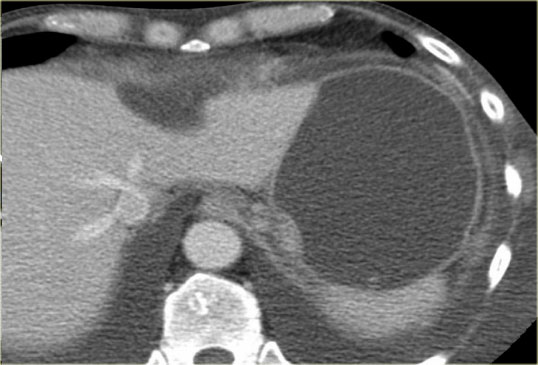
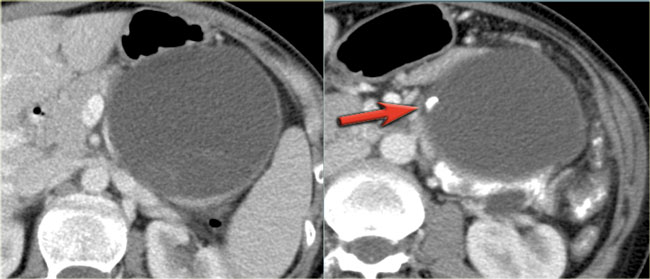
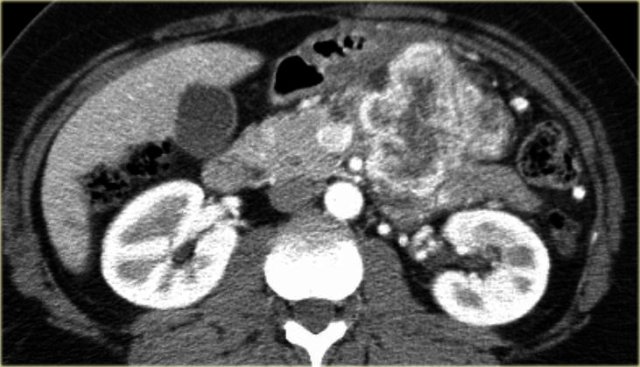
CT-image of a 51 year old woman with a history of gallstones and abdominal pain.
There is a hypodense lesion with central calcification in the head of the pancreas.
The lesion has a lobulated contour.
Continue with the MR.
MRI better demonstrates the morphologic features of the lesion (fig).
On T2WI the lesion is multicystic.
Note the central low signal due to the central scar with calcifications.
Although some of the cysts are rather large, this is still a characteristic appearance of a serous cystic adenoma (macrocystic form).
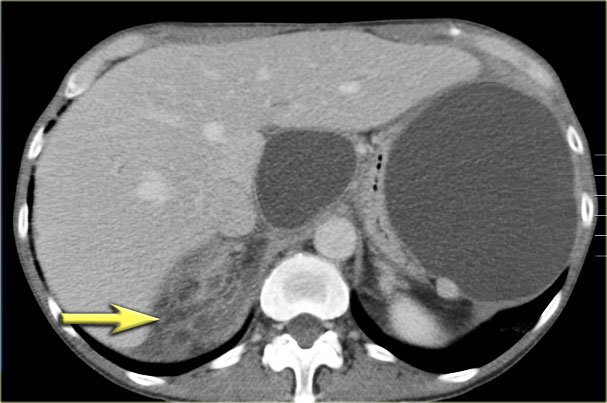
Another example of a serous cystic neoplasm (Fig).
The contrast-enhanced image on the right shows a hypodense lesion with central calcification in the body of the pancreas and subtle enhancement of septations
Notice that on CT it is very difficult to appreciate the cystic nature of these lesions and you might think that you are dealing with a pancreatic adenocarcinoma.
MRI will easily demonstrate the cystic nature of these lesions (fig).
The T2WI with fatsat nicely demonstrates a lobulated hyperintense lesion with central scar, which is characteristic of a SCN.
It may be difficult to differentiate a serous microcystic adenoma from a branch-duct IPMN or intraductal papillary mucinous neoplasm.
IPMN is always connected to the pancreatic duct, but in many cases it is difficult to see the connection.
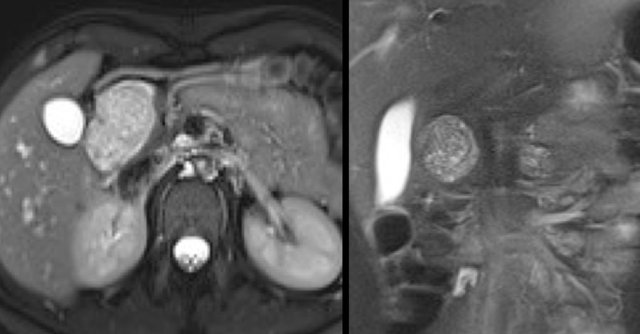
The image shows a T2WI of a 71 year old man with a history of weight loss and nondescript upper abdominal complaints.
This was initially thought to be a branch-duct IPMN, but turned out to be a SCN.
Notice the central hypointensity.
This is scar tissue in a SCN.
Notice also the characteristic lobulated surface.
 Serous Cystic Neoplasm. Courtesy Koenraad Mortel, Dept Radiology, Brigham and Women's hospital, Boston
Serous Cystic Neoplasm. Courtesy Koenraad Mortel, Dept Radiology, Brigham and Women's hospital, Boston
Here another example of a serous cystadenoma.
Notice the central enhancement.
Sometimes differentiation from a hypervascular cystic neuroendocrine tumor can be difficult, but in this case the central calcifications are helpful.
Scroll through the images.
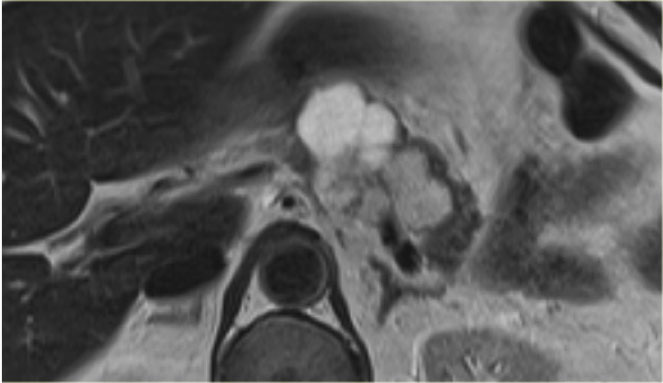
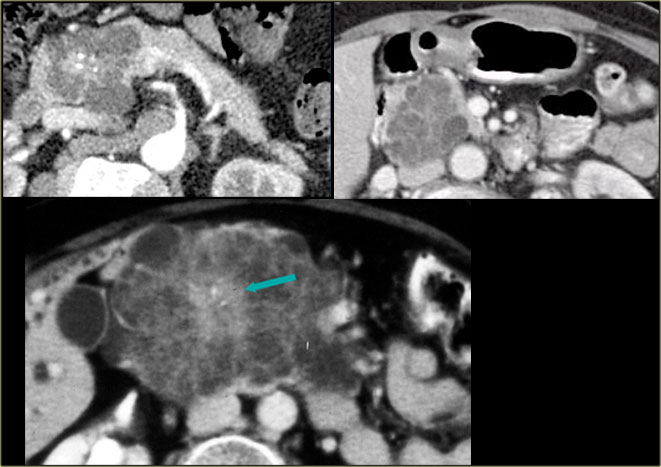
In the pancreatic tail is a cystic lesion with a central scar with calcifications (arrow).
Even though some of the cyst are larger than 2 cm, this presentation still is typical for a serous cystic neoplasm, because of the central scar, multilocular appearance and the lobulated contour.
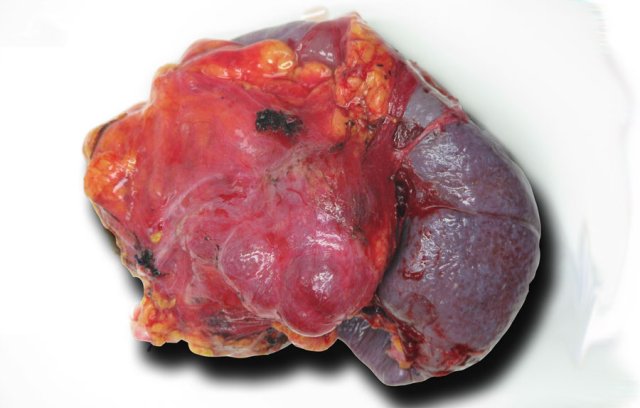
This patient had abdominal complaints which were attributed to the tumor, which was resected and proved to be a serous cystic neoplasm.
This is the resected specimen.
The tumor was attached to the spleen, which also had to be resected.
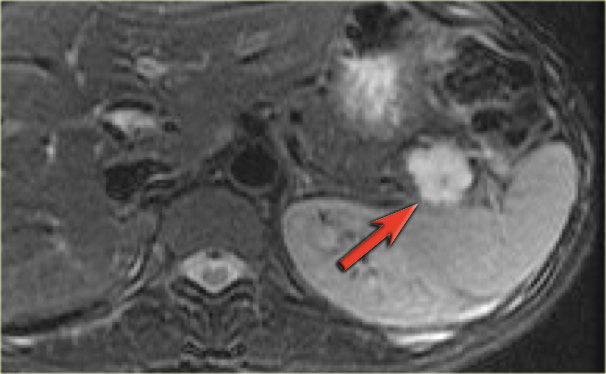
Here another typical serous cystic neoplasm (fig).
There is a microcystic lesion with a central scar in the pancreatic head.
This patient felt a mass in her abdomen.
Otherwise there were no complaints.
Because resection would mean extensive surgery, it was decided to follow the lesion.
During 5 year follow up there was no growth and the patient has no symptoms otherwise.
Mucinous Cystic Neoplasma
Key findings:
- Premalignant tumor - may transform into a mucinous cystadenocarcinoma
- Exclusively seen in women - Typically in 'Mother' - median age: 40-50 years
- Macrocystic with thick wall septations.
- Peripheral calcifications seen in 25%. This finding allows you to make a specific diagnosis.
- Location in the tail and body of the pancreas (95%).
- Most are symptomatic, presenting with nondescript abdominal pain
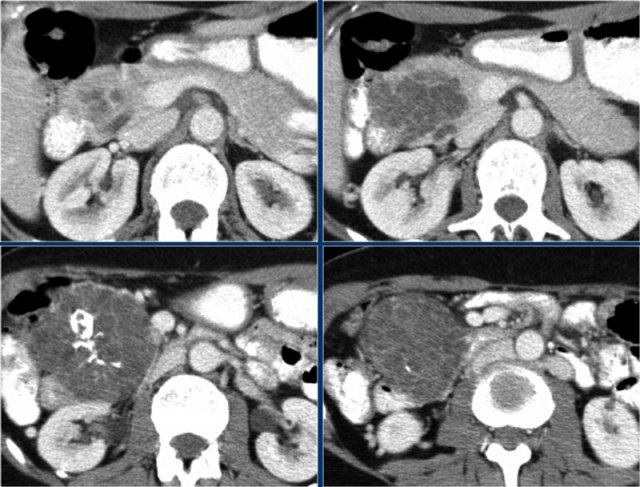
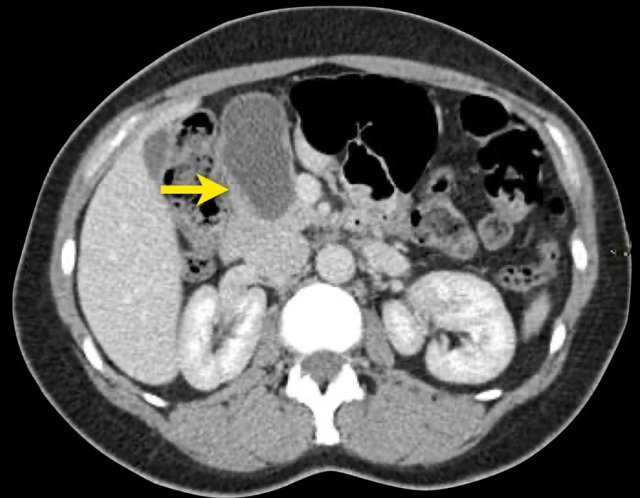
CT-images of a 32 year-old female with pain in the upper left quadrant radiating to the back.
There is a large cyst in the pancreatic tail with peripheral calcification.
There is subtle septation as seen on the left image and wall thickening.
You may have to enlarge the image to see the septation.
A specific diagnosis of a MCN can be made.
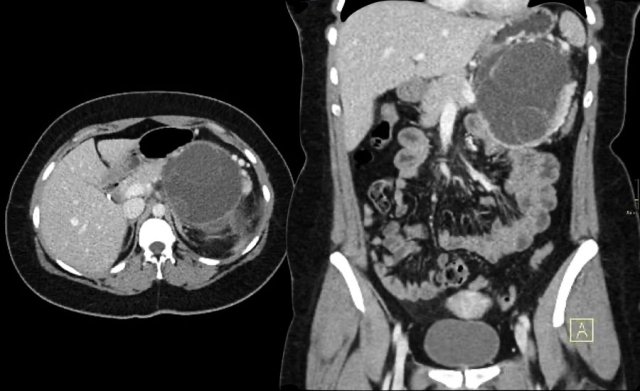
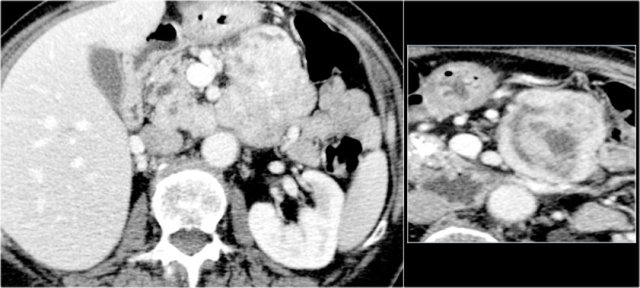
CT-image of a 46 year old female with vague right abdominal complaints.
The imaging findings are:
- septated cyst of 7 cm in the pancreatic head.
- smooth capsule.
- no lobulation.
- no connection to the pancreatic duct.
MRI revealed a septated pancreatic head cyst of 7 cm with a smooth capsule without lobulation and no connection to the pancreatic duct.
Surgery showed a low grade mucinous cystadenoma with ovarian stroma.
CT images of a 30 year old female with a history of a biliary pancreatitis and cholecystectomy.
She had sudden increased left abdominal pain.
US showed increased size of a cystic lesion, which was diagnosed as a pseudocyst.
The CT however showed a non-lobulated cystic lesion in the pancreatic tail with internal enhancing septation without connection to the pancreatic duct (fig).
Continue with the MRI...
The T1W-image post gadolinium better depicts the internal septations.
Pancreatic tail resection revealed a 14 cm mucinous cystadenoma including ovarian stroma.
Intraductal Papillary Mucinous Neoplasm
key findings:
- Mucin producing tumor in main pancreatic duct or branch-duct.
- Location: pancreatic head >> tail and corpus.
- Must have communication with pancreatic duct. Best seen with MRCP.
- Can be multifocal.
- Main-duct IPMN has imaging features distinct from branch-type.
- Branch-duct type can look like other cystic neoplasms
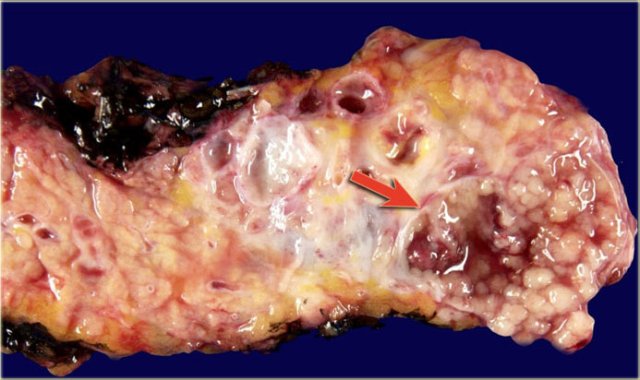
Macroscopic specimen of a IPMN showing mucinous tumor, with extensive mucin producing papilary neoplasm (arrow).
Main-duct IPMN
On imaging Main-duct IPMN is usually distinct from branch-duct IPMN, but sometimes there is a mixed type.
Scroll through the images of a large main duct and branch-duct IPMN.
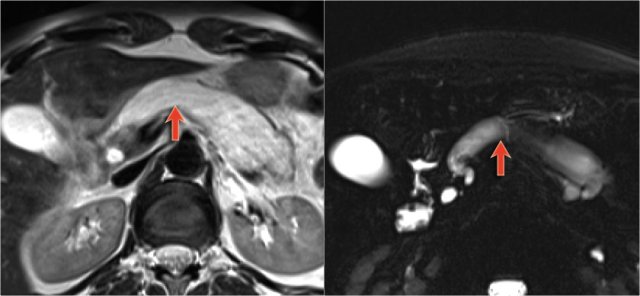
There is obstruction of the common bile duct with dilatation of the intrahepatic bile ducts (blue arrows).
Notice the extremely widened main pancreatic duct (red arrow).
Normal T2WI and heavily T2WI with fatsat of a large main duct IPMN with extremely dilated pancreatic duct.
This patient presented with pancreatitis.
The MRCP shows both a main-duct aswell as a branch-duct IPMN (arrow).
IPMN is a lesion with malignant potential.
Signs of malignancy are:
- Pancreatic duct > 8 mm - as in this case.
- Solid node in duct.
- Mass around the pancreatic duct.
- Enlarged choledochal duct.
CT-images of an IPMN with a dilated pancreatic duct (blue arrows).
Notice enhancing solid nodule in the pancreatic head (red arrow).
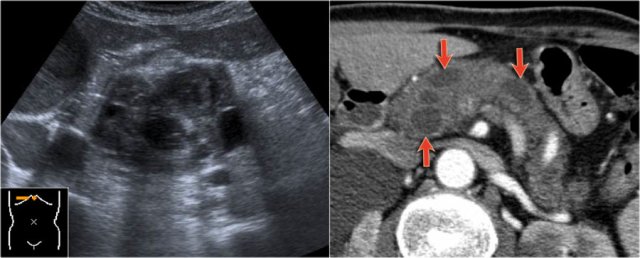
Continue with the ultrasound-image.
The US-image shows a large branch-duct component within the pancreatic head.
Branch-duct IPMN
The CT-image shows a hypodense lesion in the pancreatic head.
This could be an adenocarcinoma, but the low density makes you think of a cystic tumor.
The microcystic appearance raises the possibility of a serous cystic neoplasm although there is no calcified scar.
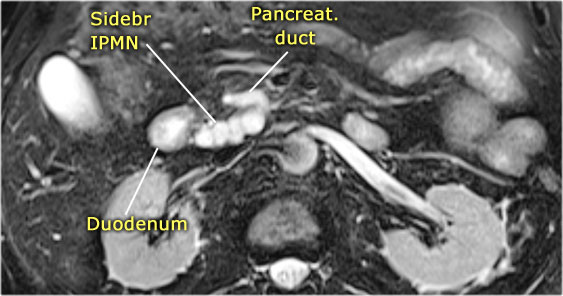
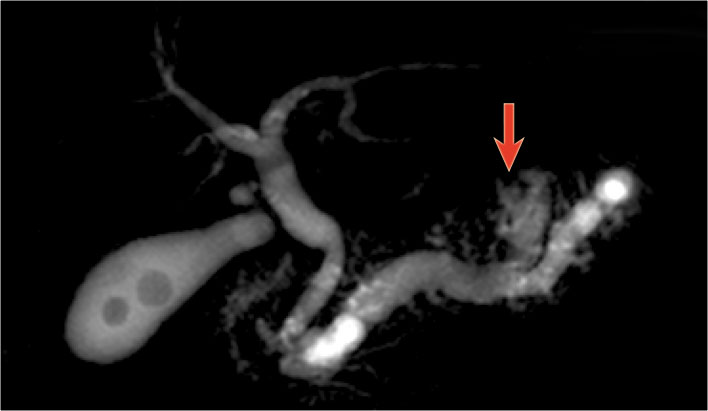
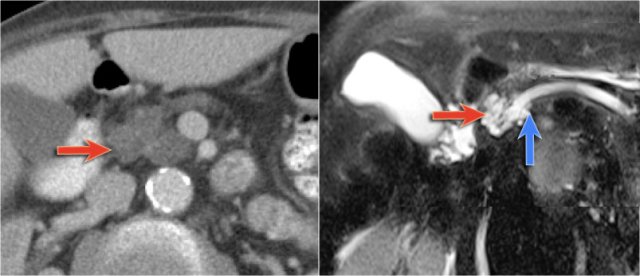
On MRCP the cystic nature is better appreciated and there is a connection to a widened duct (blue arrow).
A detail nicely demonstrates that some of the mucus-filled branches are seen in cross-section and some longitudinally.
In a 73 year old male a hypoechoic lesion was found in the pancreatic body, that looked like a cystic lesion.
CT also identifies the lesion but isn't of much help.
Continue with the MRI.
The heavily T2WI nicely demonstrates the multicystic lesion with the connection to the pancreatic duct.
This was diagnosed as a branch-duct IPMN.
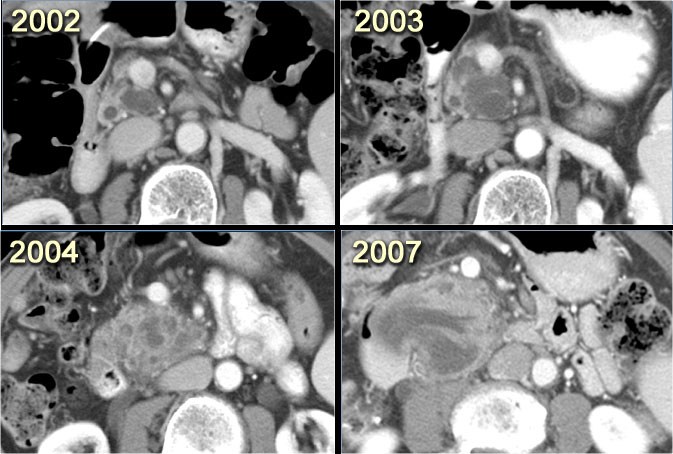
CT-images of a patient with a branch-duct IPMN who choose not to have surgery.
Over time growth of the tumor is seen with dilatation of the main duct indicating malignant transformation.
Sometimes it takes 5-8 years before a transformation is seen.
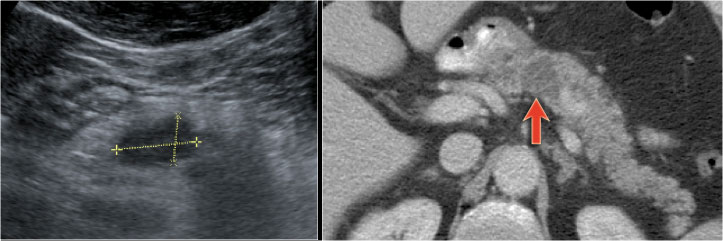
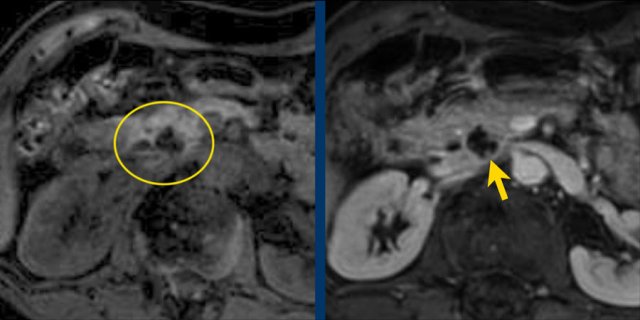
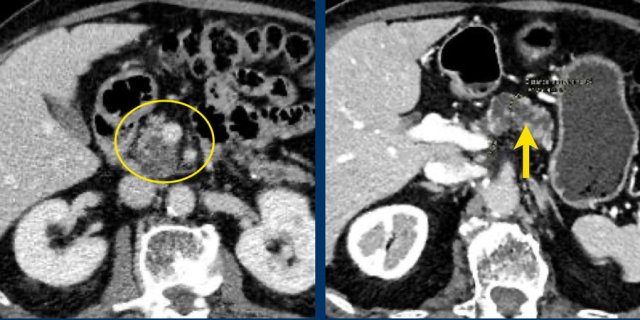
Another branch-duct IPMN found on screening with two nodules (circle and arrow).
T1W-images with fatsat before (left image) and after contrast (right image).
EUS with contrast agent revealed 2 foci without enhancement most likely mucus plugs.
6 years later the cyst was unchanged.
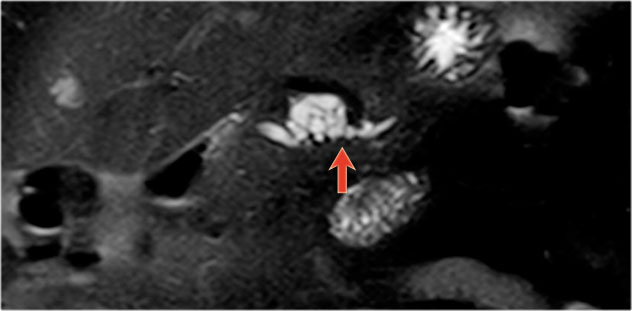
75 year old male with a 2.7 cm side branch IPMN in the pancreatic head (circle).
During follow up one year later dilatation of pancreatic duct was seen.
EUS showed a resectable adenocarcinoma.
Uncommon Neoplasms with specific findings
Solid Pseudopapillary Neoplasm
key findings:
- Very uncommon neoplasm seen in women 20-30 years (daughter).
- Solid and cystic neoplasm with capsule and with early 'hemangioma-like' enhancement.
- Sometimes intratumoral hemorrhage
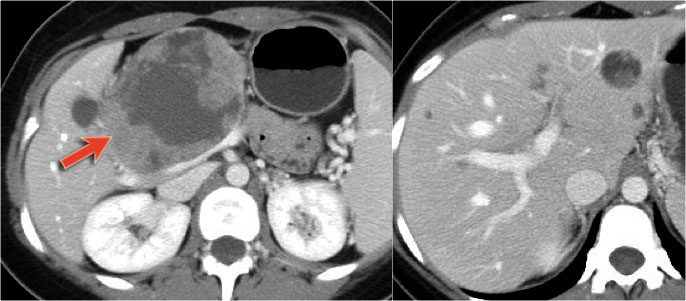
CT-images of a 26 year old woman with a large mass in the pancreatic head and metastases in the liver.
In the center there is lack of enhancement due to cystic or necrotic degeneration.
Solid tumor with cystic components in a 16 year old female diagnostic of solid pseudopapillary tumor.
Neuroendocrine tumor with cystic degeneration
key findings:
- Non-functioning endocrine neoplasm
- Also called islet cell tumor
- Hypervascular with ring-enhancement. This is unlike serous cystic neoplasms that enhance from the center and more solid)
CT-images of a 61 year old woman with weight loss.
There is a large mass in the body of the pancreas that is hypervascular, unlike an adenocarcinoma, with some cystic or necrotic parts.
CT-image of a neuroendocrine tumor with central necrosis.
Sometimes this can simulate a cystic component.
Notice the peripheral enhancement.
Report and Management
In the table a checklist of what to mention in the report and the relative and absolute indications for resection according to the European evidence-based guidelines on pancreatic cystic neoplasms (2).
Continue with the guidelines for management.
The frequency of imaging follow-up depends on the presence of indications and fitness for surgery as can be seen in the table.
Although these management guidelines apply to IPMN, in general practice we use these criteria also for pancreatic cysts of unknowm origin and suspected mucinous cystic neoplasms. However in suspected Mucinous Cystic Neoplasm a cyst size ≥ 4 cm is an absolute criterium for resection, whereas for IPMN it is a relative indication.
Imaging protocol
The initial MRI should be done using a dedicated pancreatic protocol (tab).
A possible follow-up protocol for lesions < 3 cm may consist of coronal and axial T2 single shot sequences and T1 weighted precontrast and no post contrast.
Possibly adding diffusion weighted images to minimize risk of missing a concomitant pancreatic carcinoma.
We have chosen to follow-up cysts smaller than 3 cm without intravenous gadolinium with the rest of the sequences the same. If we find a possible new nodule we would return the patient and repeat the MR scan with IV gadolinium to evaluate for enhancement.