RECIST 1.1 - the basics
Response Evaluation Criteria In Solid Tumors
Fokko Smits, Martijn Dirksen and Ivo Schoots
Radiology Department of the Erasmus MC in Rotterdam and the Isala hospital in Zwolle, the Netherlands
Publicationdate
RECIST 1.1 is a standard way to measure the response of a tumor to treatment.
In this article we will discuss the basics of RECIST.
The criteria to determine whether a tumor disappears, shrinks, stays the same or gets bigger are complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).
More information is given in the article RECIST 1.1 and more in which we will discuss RECIST 1.1 in more detail and discuss other response systems.
Introduction
RECIST is a standard way to measure the response of a tumor to treatment.
CT is the preferred modality for the baseline study.
The baseline scan should be done within 4 weeks before treatment starts and slice thickness ⩽ 5 mm and i.v. contrast are mandatory.
Choose target lesions (max 5) that are easy to measure and calculate the sum of the longest diameters (SLD).
Identify non-target lesions like ascites or pleural fluid that are not suited for exact measurements, but that can be followed.
In the follow up study compare the SLD of the target lesions and determine the presence or abcense of the non-target lesions and look for new lesions.
Then determine whether there is complete response, partial response, stable disease or progressive disease.
Any new lesion means progressive disease.
The response criteria can be seen in the table.
The sum of longest diameters of the target lesions (SLD) in the follow up scan has to be compared with the baseline and the smallest SLD during treatment, called the ‘nadir'.
The absolute increase of SLD should be ≥ 5mm to be called progressive disease.
Target lesions
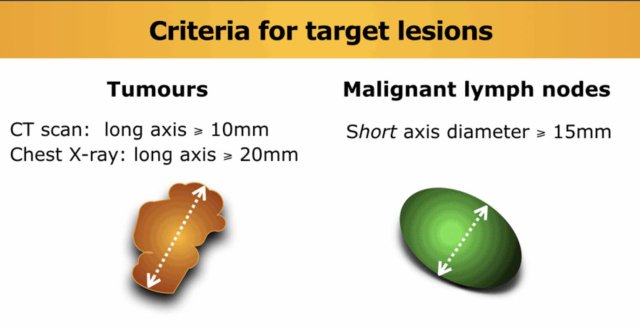
Tumors
Choose preferably large well-described lesions to measure with a longest diameter ≥ 10 mm, a maximum of two per organ and a maximum of five for the whole study.
Lymph nodes
Lymph nodes can be used as target lesions provided that the maximum short axis diameter exceeds 15 mm.
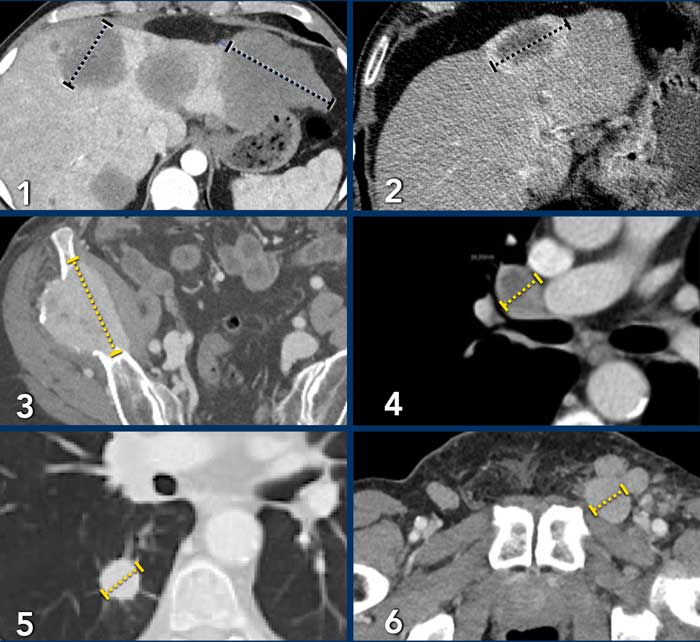
Examples of target lesions:
- Liver metastases: maximum of 2 measurements per organ.
- Include hypervascular ring for measurement.
- Bone metastasis, only soft tissue component is suitable for measurement as target lesion. Sclerotic lesions are not suited for target lesion.
- Mediastinal lymph node: short axis ⩾ 15mm.
- Lung metastasis ⩾ 10mm.
- Inguinal lymph node short axis ⩾ 15mm.
Lymph nodes <10 mm are regarded as normal.
Lymph nodes 10-14mm are regarded as pathologic, but not suited for target lesions.
They can be used as non-target lesions.
When target lymph nodes decrease to a normal size (<10 mm), their measurements still have to be included in the sum of the longest diamters (SLD).
Special notes on defining target lesions
- Bone lesions
In lytic or mixed lytic-blastic bone lesion, only the identifiable soft tissue component is suitable for measurement as target lesion.
Blastic lesions are non-measurable. - Cystic lesions
Simple cysts should not be regarded as malignant lesions or serve as target lesions.
Suspected cystic or necrotic metastases may apply, however if concomitant solid metastases are present rather use those. - Loco-regional therapy
Lesions located in an area, that has been subjected to loco-regional therapy (eg. radiotherapy), are considered non-measurable.
Sum of Longest Diameters
Here an example of a 28-year-old male with a neuroendocrine carcinoma of the appendix.
There are 5 lesions suited for measurement:
- In the liver there are two metastases that are suited for measurement (1 and 2).
- In the porta hepatis there is a lymph node with a short axis > 15mm (3).
- There are two peritoneal metastases (4 and 5).
The SLD = diameter of 1+2+3+4+5.
Non-target lesions
 During follow up there is unequivocal progression of lymph node metastases, which were choosen as non-target lesions
During follow up there is unequivocal progression of lymph node metastases, which were choosen as non-target lesions
Non-target lesions are:
- Tumor lesions < 10 mm
- Lymph nodes ≥ 10 -15 mm
- Truly non-measurable disease: eg. leptomeningeal disease, ascites , effusions, inflammatory breast disease, and lymphangitic involvement of skin or lung.
- Any supernumerary lesions that meet the measurability criteria when the maximum number of target lesions has been reached.
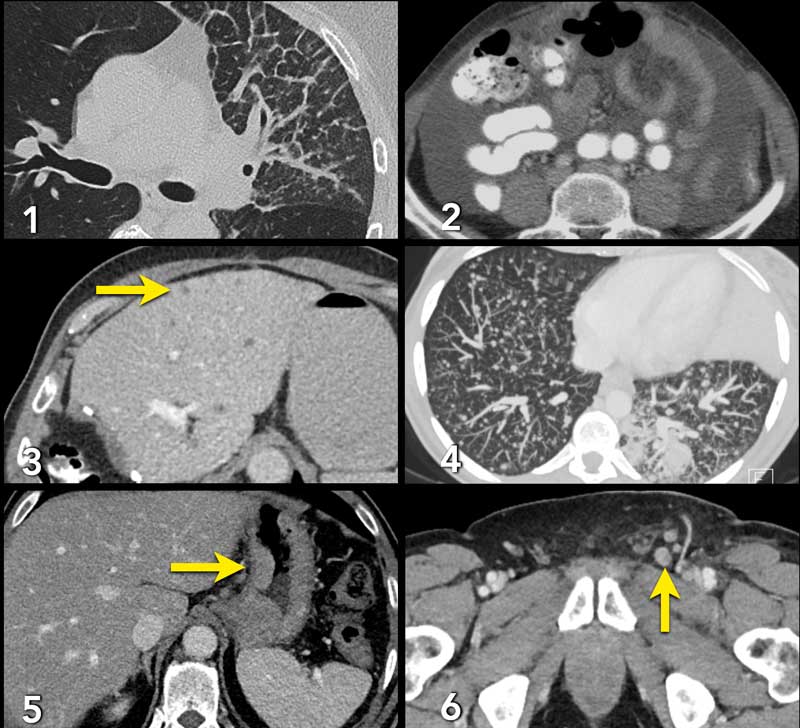
Examples of non-target lesions:
- Pulmonary lymphangitis carcinomatosa.
- Ascites.
- Livermetastases too small for measurement as target lesion, but can be used as non-target lesions.
- Milairy lung metastases.
- Gastric carcinoma not suited for exact measurement, because the tumor will not present exactly the same in a follow up scan due to position of the stomach and peristalsis.
- Inguinal lymph nodes too small for target lesions (<15mm).
You do not measure non-target lesions, but make a good estimate.
In the follow up there are 3 possibilities:
- disappearance
- no disappearance (about the same)
- unequivocal progression (fig)
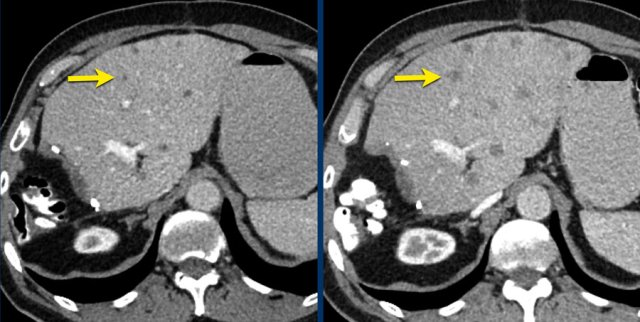
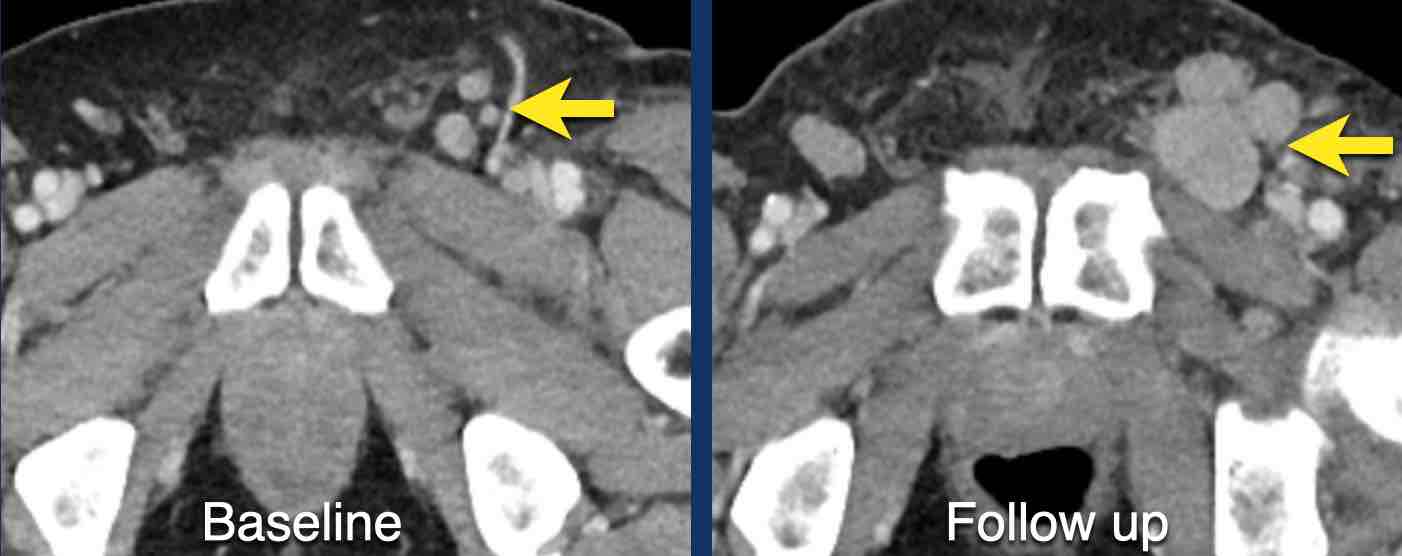
Here another example of progression of non-target lesions.
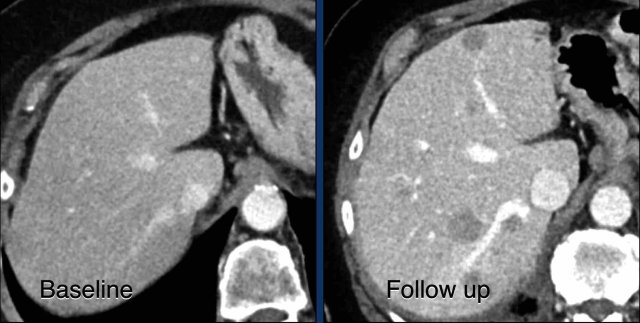
CT images in a 73-year-old male with progressive liver metastases of colorectal carcinoma.
At baseline the liver metastases were too small to be used as target lesions and consequently they were used as non-target lesions.
At follow up there is unequivocal progressions.
Unequivocal progression of non-target lesions means progressive disease, even if there is partial response or even disappearance of the target lesions.
New lesions
Any new lesion means progressive disease.
Do consider “new” lesions in an area of the body that was not imaged during baseline (for example brain metastases) as truly “new”, thereby forcing overall response to progressive disease.
CT-images in a 81-year-old female with endometrial carcinoma and occurrence of new liver metastases during treatment with chemotherapy.
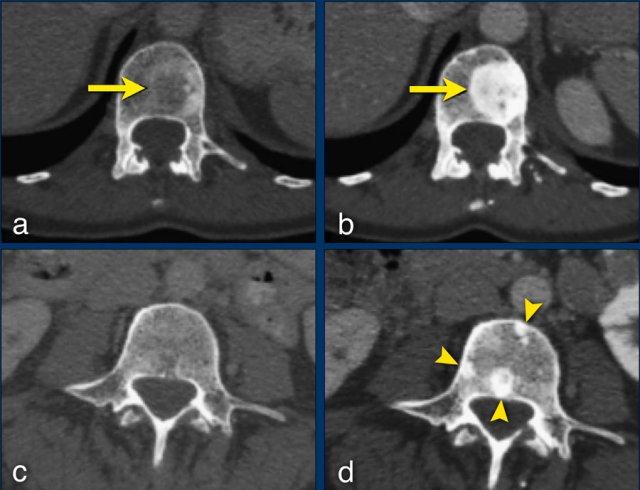
Any new lesion means progressive disease, but not every newly detected lesion is always a true new lesion.
In osteolytic bone metastases it can be difficult to determine if a sclerotic lesion that is detected during follow up is truly a new lesion.
The sclerotic bone lesions in b and d are not new metastases but an osteoblastic reaction to the therapy.
Response assessment
This table is the same as the table in the introduction, but demonstrates the overall treatment response with varying responses in the three lesions categories.
Special remarks
Do's
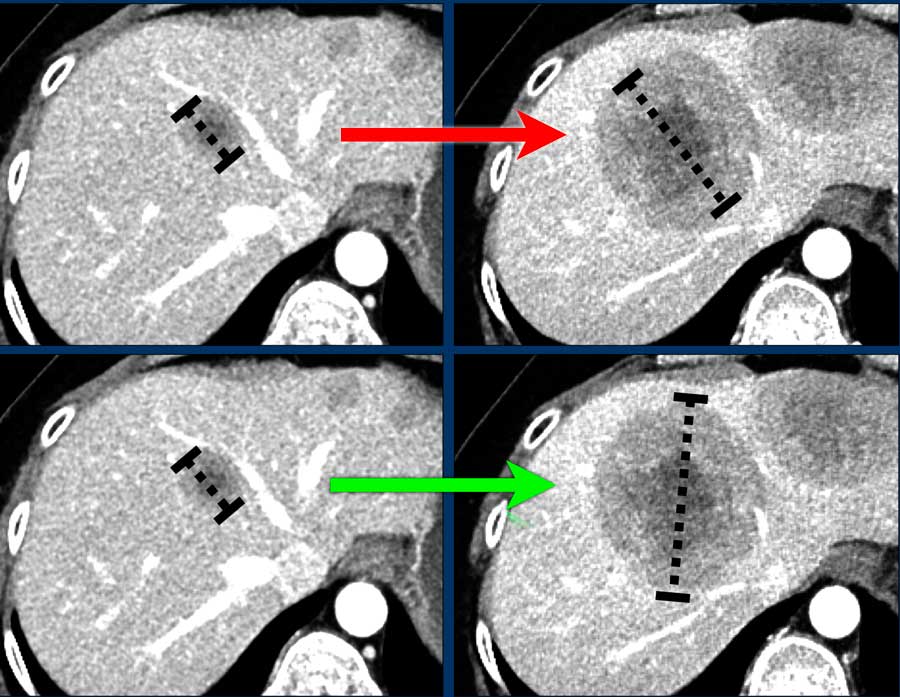
- Do measure the longest diameter if the orientation of longest diameter varies during follow-up (fig).
- Do consider “new” lesions in an area of the body that was not imaged during baseline (for example brain metastases) as truly “new”, thereby forcing overall response to progressive disease.
- Do measure the short axis of mesothelioma.
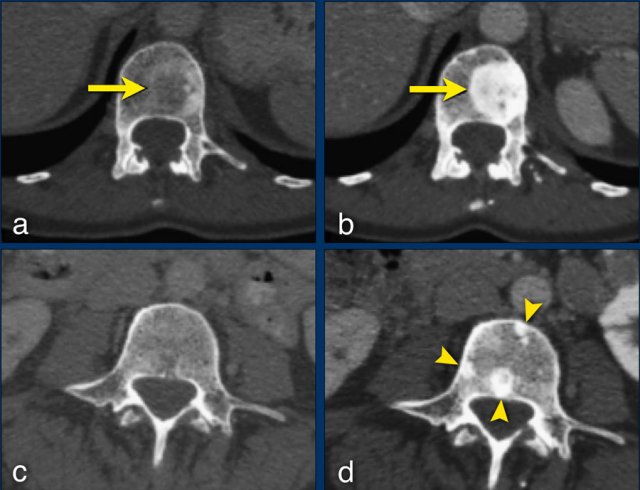 The sclerotic bone lesions in b and d are not new metastases but an osteoblastic reaction. Courtesy Els van Persijn van Meerten.
The sclerotic bone lesions in b and d are not new metastases but an osteoblastic reaction. Courtesy Els van Persijn van Meerten.
Don'ts
- Don't regard “new” sclerotic bone lesions as progressive disease as they may represent filling-in of previously lytic lesions that were not detected at baseline (fig).
- Don't change target lesions or non-target lesions during follow-up studies: “once a target lesion, always a target lesion”
- Don’t change measurements on previous studies
- Don't measure lesions after treatment by RF ablation or cryoablation, because the ablated area is often larger than the original tumor.
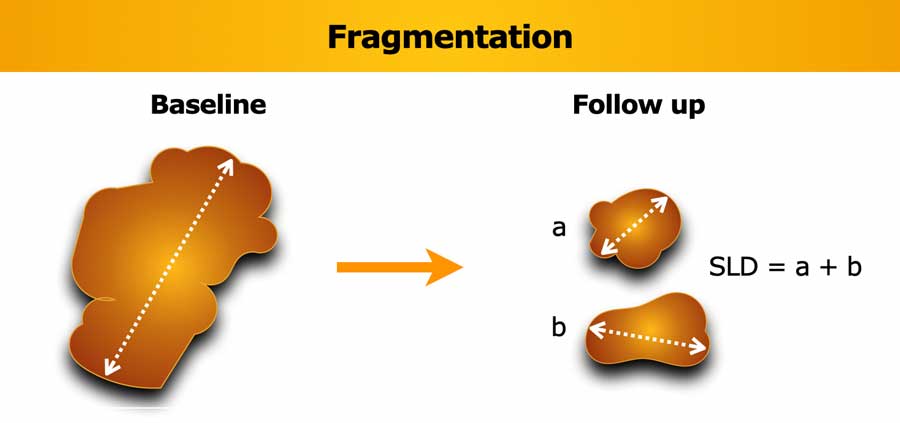
Fragmentation of lesions
If a lesion breaks into separate fragments between baseline and follow-up, the sum of longest diameters (SLD) of the fragments should be calculated.
Obviously, the short axis diameter is measured for lymph nodes.
Too small to measure
At each response evaluation each target lesions should be measured, even when they are very small (e.g. 2 mm).
If a target lesion has disappeared record a measurement of 0 mm.
If the lesion is too small to measure (faintly seen and not possible to give an exact measurement) assign a default value of 5 mm to prevent false responses or progression (derived from the 5 mm CT slice thickness).
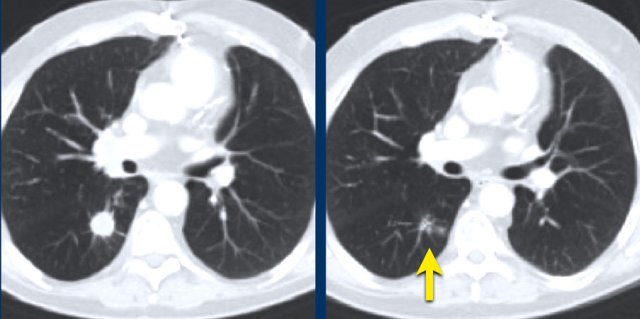
The images are of a patient with a primary lung tumour.
At baseline, the longest diameter is well above 10 mm, therefore this was assigned as a target lesion.
During follow-up the long-axis diameter dropped below 10 mm, which is the lower limit for considering a lesion as target lesion.
However, since this is a follow-up measurement, the target lesion still counts up to the sum of the diameters (SLD) and a default value of 5mm was assigned.
Cavitating lesions
Cavitation can occur during treatment.
Cavitating lesions should be continuously measured in their longest diameter.
A different assessment can be provided if the sum of the longest diameters does not adequately correspond to the patients response assessment.
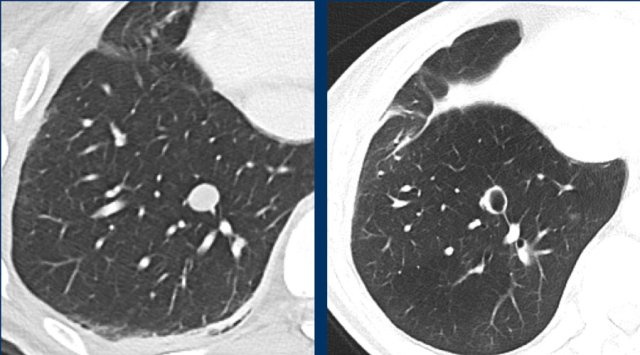
These CT images are of a 32-year-old male with a pulmonary metastasis of a malignant peripheral nerve sheath tumour.
Cavitation occured after treatment with pazopanib, but the size remained the same.
Although the size remains the same, a remark can be made in the report, that the actual tumorvolume has decreased.