LI-RADS
Liver Imaging Reporting And Data System
Frederieke Elsinger*, Christopher Lunt, Alison Harris and Silvia Chang
Luzerner Kantonsspital* and Vancouver General Hospital
Publicationdate
The Liver Imaging Reporting and Data System (LI-RADS) is a classification system for liver lesions which is used in patients with liver cirrhosis and chronic HBV without cirrhosis, because these patients have an increased risk of hepatocellular carcinoma (HCC).
The LI-RADS category reflects the probability of HCC and is based on the typical CT and MR-findings in HCC.
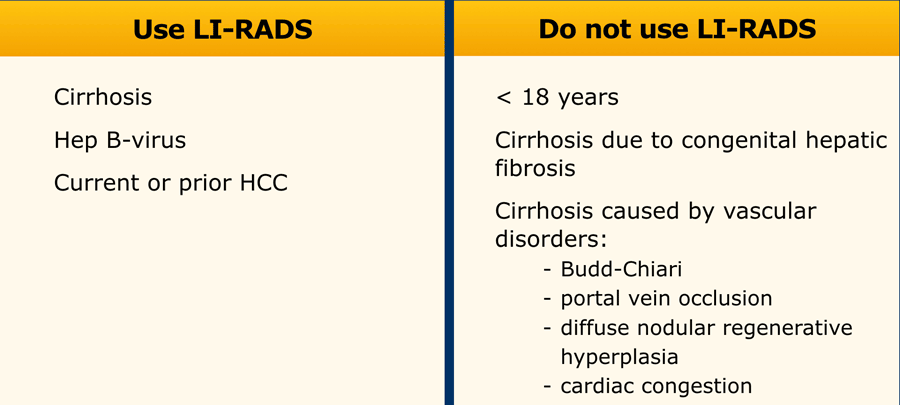
LI-RADS is not meant to be used in patients <18 years or patients with cirrhosis due to congenital hepatic fibrosis or due to vascular disorders, because these patients have a lower chance of developing HCC.
Press ctrl+ for larger images and text on a PC or ⌘+ on a Mac.
Single images can be enlarged by clicking on them.
Introduction
LI-RADS major features
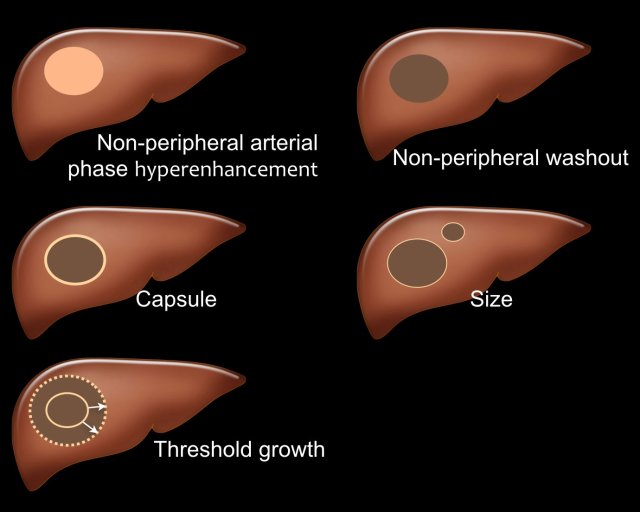
There are five major features which are typically seen in HCC in patients with livercirrhosis and chronic hepatitis B virus infection (fig).
- Arterial phase hyperenhancement (APHE)
APHE is non-rim arterial hyperenhancement of a lesion which is greater than the enhancement of the surrounding liver. Rim enhancement is not a feature of HCC. - Non-peripheral washout
Decrease in attenuation or intensity from earlier to later phase, resulting in hypoenhancement in the portal venous or delayed phase. - Capsule
Smooth, uniform border surrounding all or most of an observation. - Size
A large lesion has a greater chance of being a HCC than a small lesion. - Threshold growth
Threshold growth is increase in size of 50% or more within 6 months time during follow-up imaging.
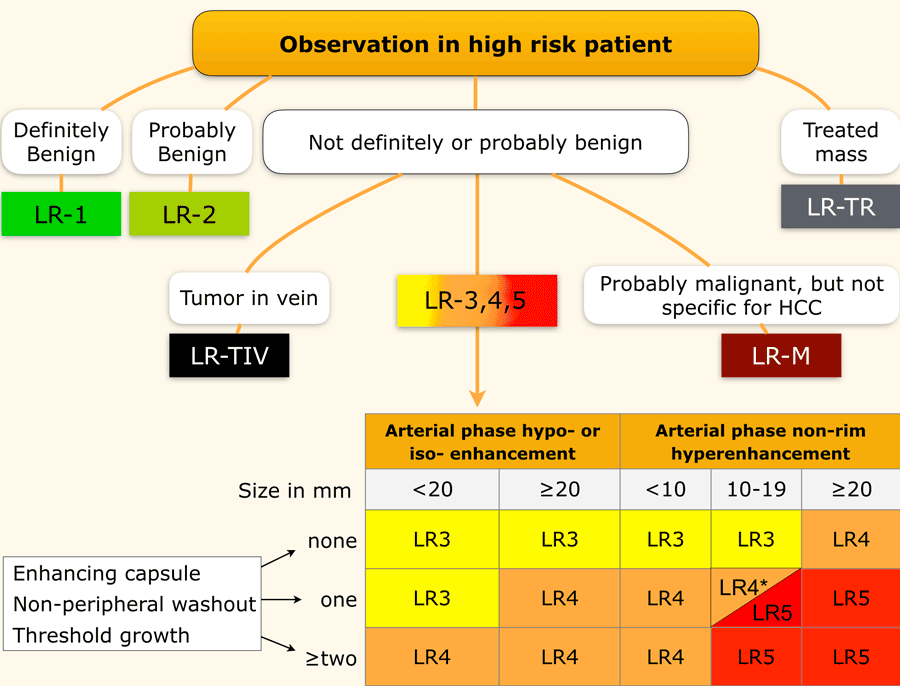
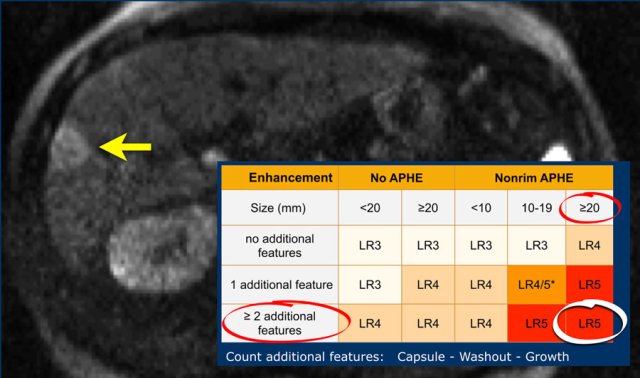
LI-RADS categories
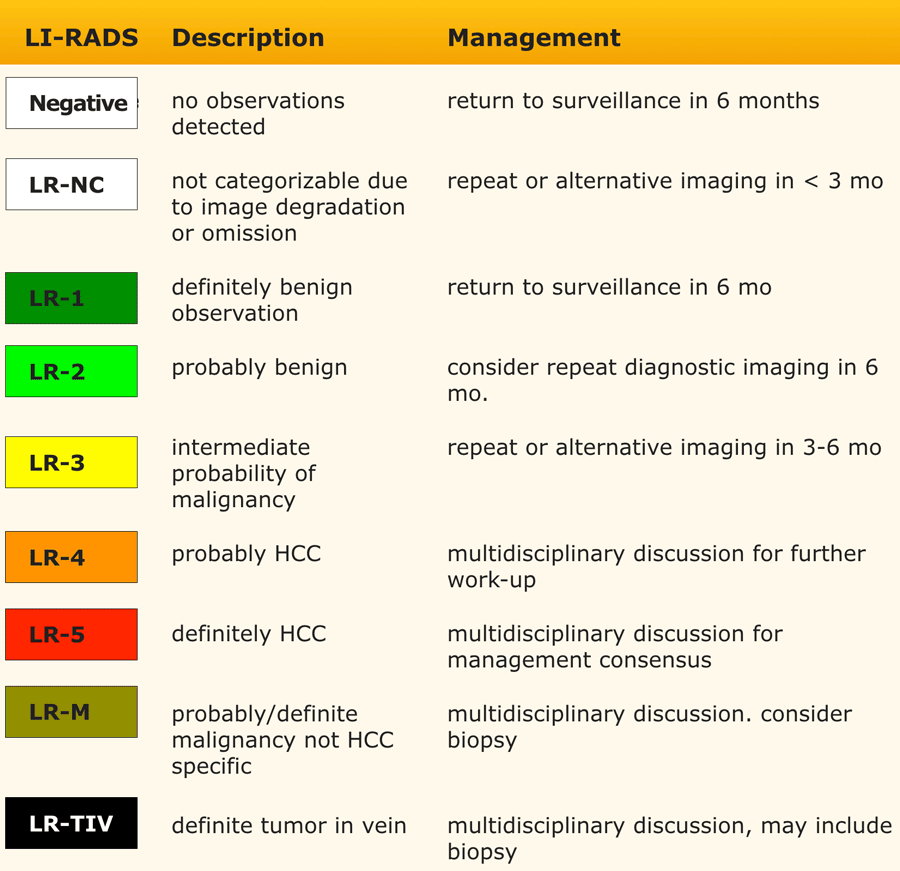
This table is an overview of all the LI-RADS categories.
The term observation is used when it is not sure, whether something is a lesion or a pseudolesion.
The LI-RADS categories reflect the probability of an observation being a HCC and ranges from LR-1 (definitely benign) to LR-5 (definitely HCC).
Use LI-RADS as follows:
- First determine whether there is enhancement.
- Then look at the type of enhancement.
Non-rim hyperenhancement is more suspicious than hypo- or isoenhancement. - Then look at the size of the lesion.
- Finally look for additional typical features of HCC like enhancing capsule, non-peripheral washout and growth of the lesion.
Additional categories are:
- LR-TIV - tumor in vein.
- LR-M - malignant but not HCC.
- LR-TR - treated HCC.
- LR-NC - non-categorizable for lesions in which the technical quality of the imaging does not allow evaluation of the major features
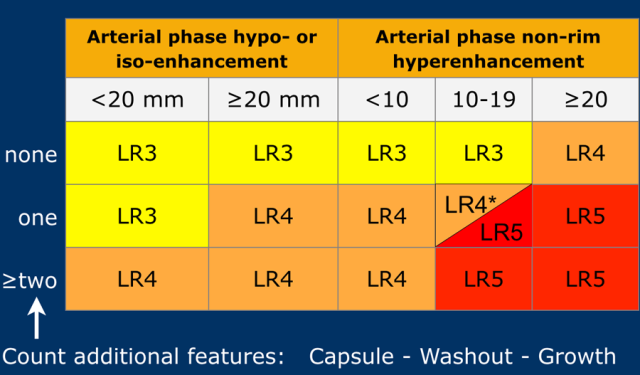
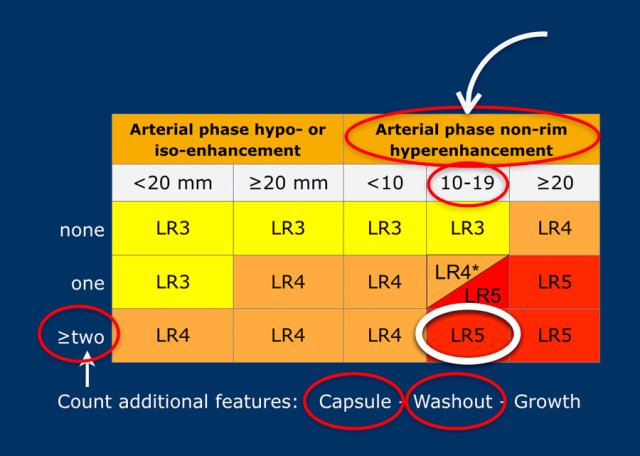
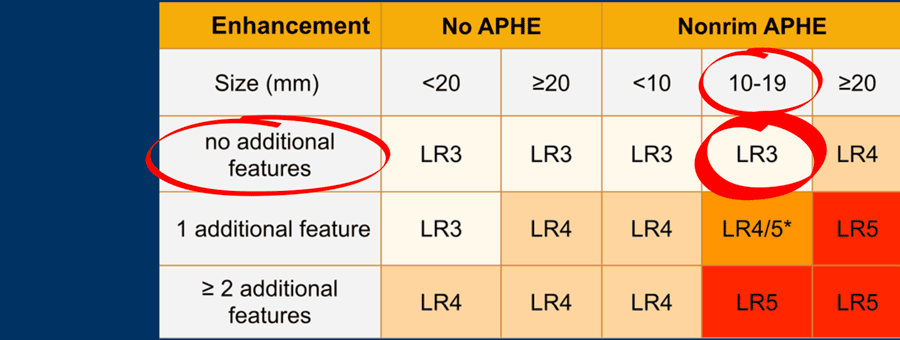
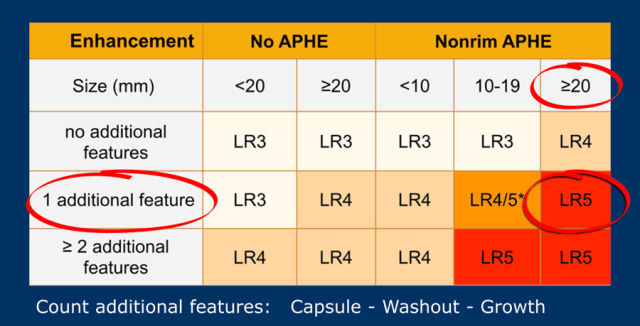
The most interesting part of the table is when we have to decide if a lesion has to be placed in category LR-3, which is intermediate probability or in LR-4, which is probable HCC or in category LR-5, which is definitely HCC.
As mentioned above, first determine if there is enhancement and if it is non-rim APHE.
Then look at the size of the observation and finally count the number of additional major features.
Suppose you detect a lesion in a patient with livercirrhosis.
There is arterial phase non-rim hyperenhancement and the size is 15mm.
There is a capsule and washout in a later phase.
This means that there are 2 additional features.
This will result in Li-Rads 5 category.
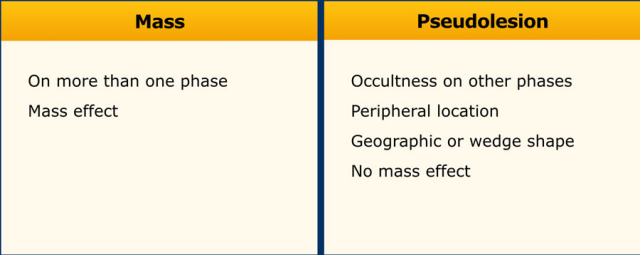
Mass versus Pseudolesion
LI-RADS uses the term ‘observations’ to describe focal abnormalities which are distinct from the background liver parenchyma.
It is preferred not to use the word ‘lesion’ or ‘nodule’ as some of these abnormalities do not represent true lesions but are the result of a perfusion alteration, a hypertrophic pseudomass or an artifact.
When to use LI-RADS
LI-RADS can only be used in patients with cirrhosis and chronic HBV infection.
LI-RADS should not be used in patients with cirrhosis caused by vascular disorders (see table).
In these patients the formation of benign hyperplastic nodules may resemble HCC on imaging and cause false positive diagnoses.
Major features
Arterial Phase non-rim Hyperenhancement (APHE)
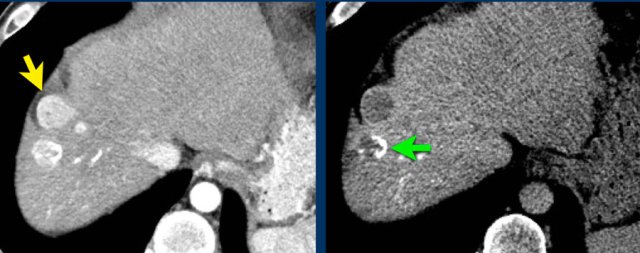
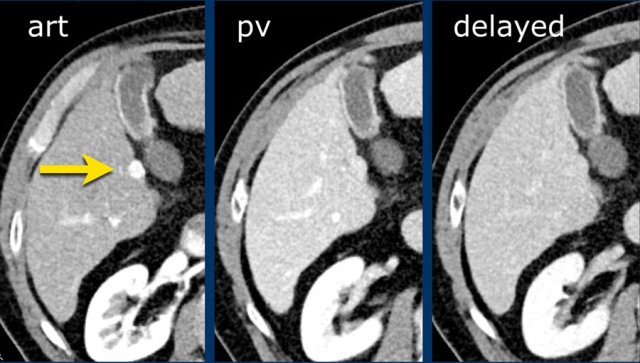
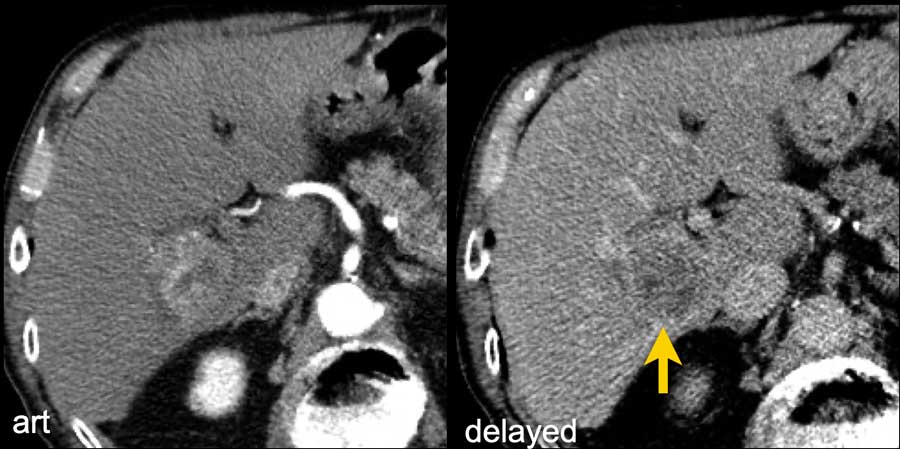
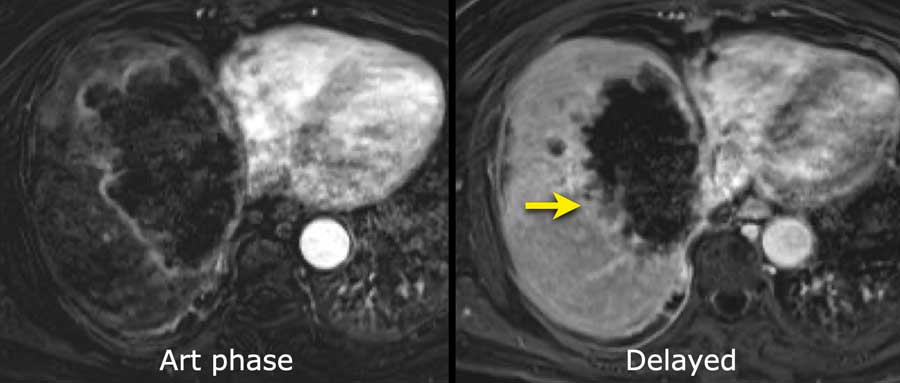
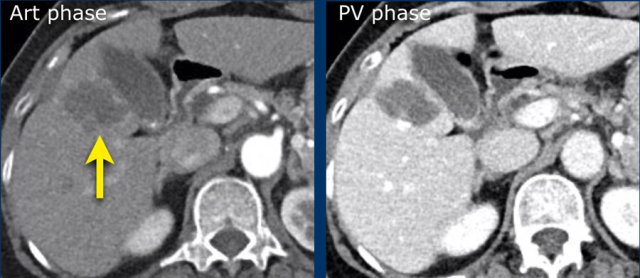
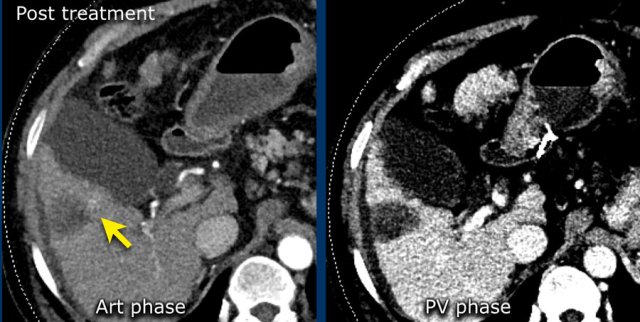
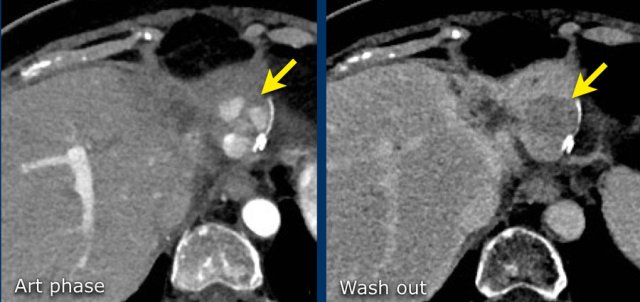
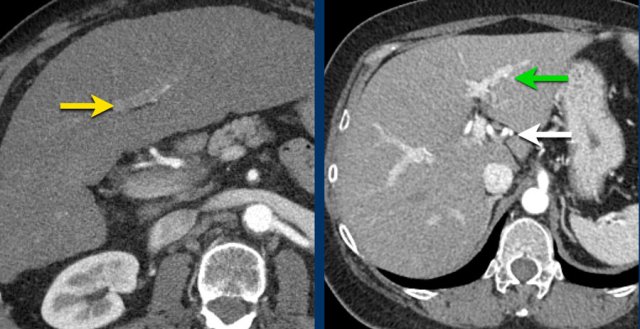
Here an image in the late arterial phase in a patient with cirrhosis.
There is an observation with non-rim hyperenhancement (yellow arrow).
In a late phase there is washout.
These are typical features of HCC.
The other lesion (green arrow) is a treated lesion, which we will discuss later.
Washout - Capsule
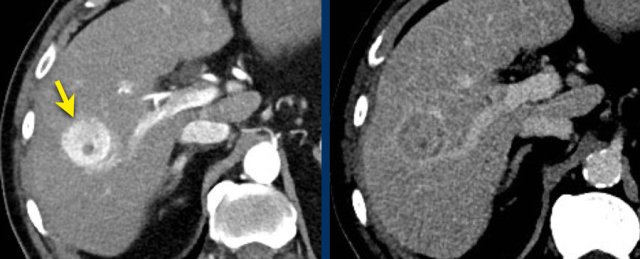
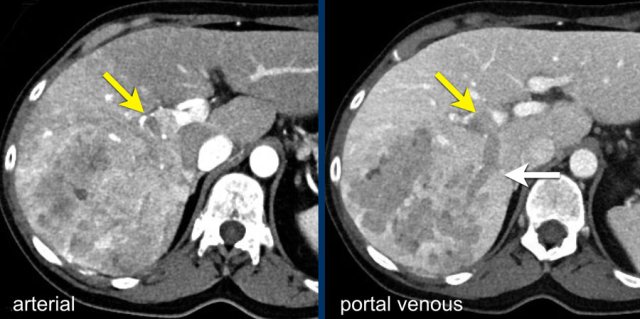
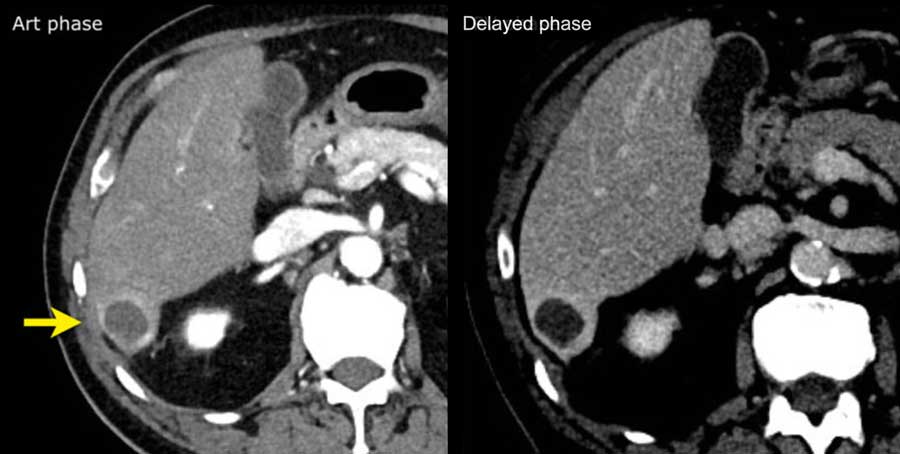
This is another patient with an enhancing lesion and washout.
Note also the enhancing capsule on the delayed phase.
A capsule is one of the major features of HCC and can be complete or partial.
A capsule should always be included within the measurement of the lesion.
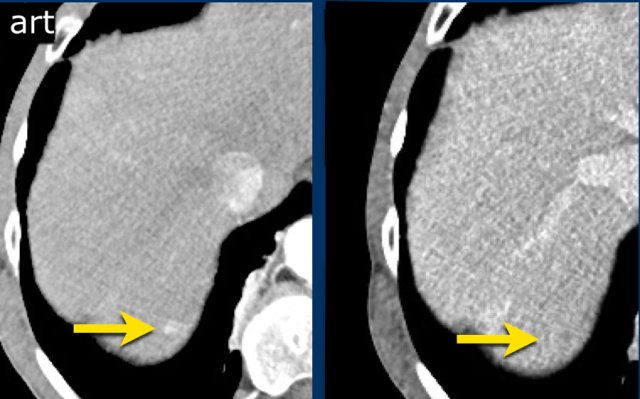
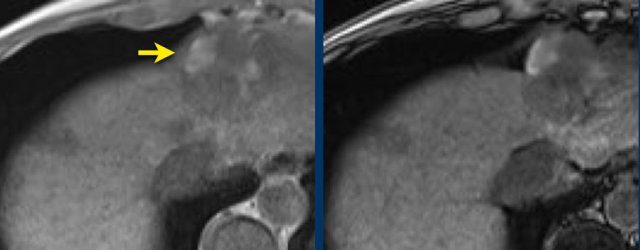
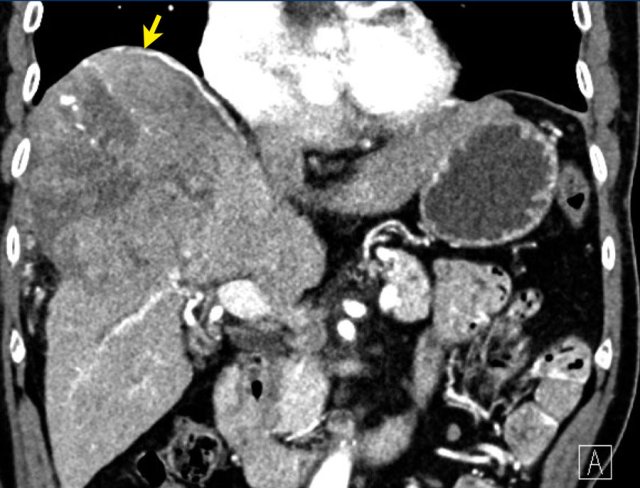
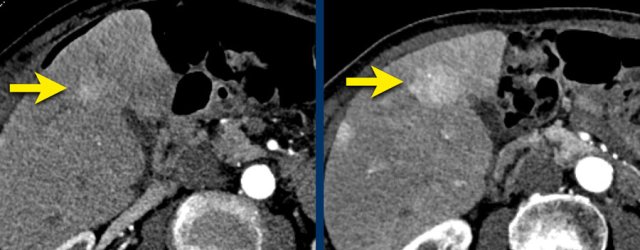 LEFT: Hyperenhancing observation detected in segment 5. RIGHT: follow up 3 months later shows growth.
LEFT: Hyperenhancing observation detected in segment 5. RIGHT: follow up 3 months later shows growth.
Size - Threshold growth
Size also determines in which category a lesion is placed.
The bigger the lesion, the higher the chance that it is a HCC.
An observation should be measured in the phase, sequence or plane in which margins are the most clear.
Measurement on the arterial phase and DWI sequence should be avoided as size could be overestimated due to summation of perilesional enhancement and anatomic distortion respectively.
Threshold growth is also an important finding.
It is defined as more than 50% growth in less than 6 months
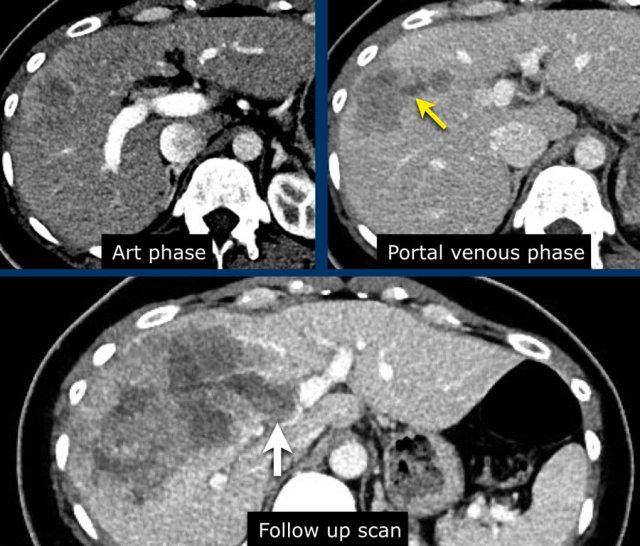
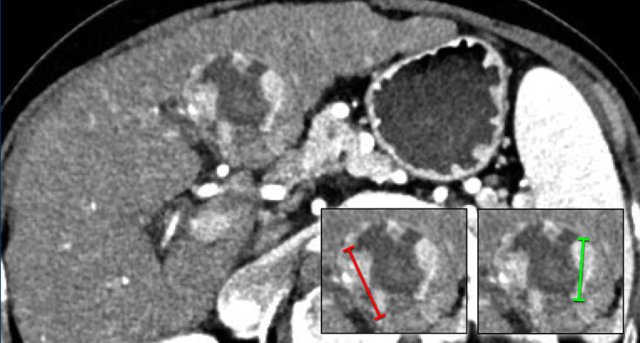
The images show an observation in segment 5 of the liver demonstrating arterial hyperenhancement.
The lesion has grown from 8 mm to 21 mm in 3 months, which means that there is threshold growth.
LI-RADS categories
LI-RADS 1 - definitely benign
Observations in this category are definitely benign.
Examples of LR-1 lesions are definite:
- Cyst
- Hemangioma
- Perfusion alteration (e.g., arterioportal shunt)
- Hepatic fat deposition/sparing
- Hypertrophic pseudomass
- Confluent fibrosis or focal scar
The images show lesions that show no enhancement in both late arterial, portal venous and delayed phase.
LI-RADS 2 - probably benign
LR-2 observations are probably benign.
Of all LR-2 lesions about 16% are HCC and 18% are malignant.
Examples of LR-2 lesions are probable:
- Cyst
- Hemangioma
- Perfusion alteration (e.g., arterioportal shunt)
- Hepatic fat deposition/sparing
- Hypertrophic pseudomass
- Confluent fibrosis or focal scar
Distinct nodules < 20 mm without malignant features (see section of ancillary malignant features) can be categorized as LR-2.
Examples are nodules which are T1 hyperintense, T2 hypointense, siderotic or hepatobiliary phase hyperintense.
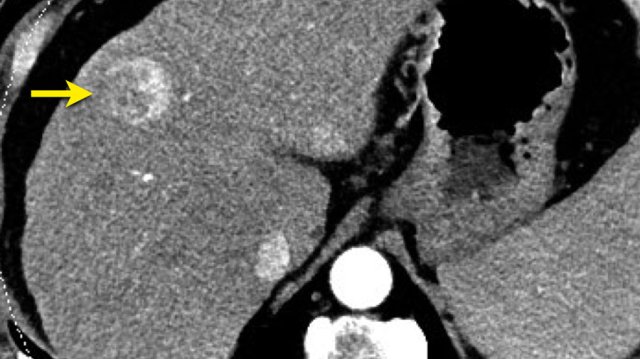
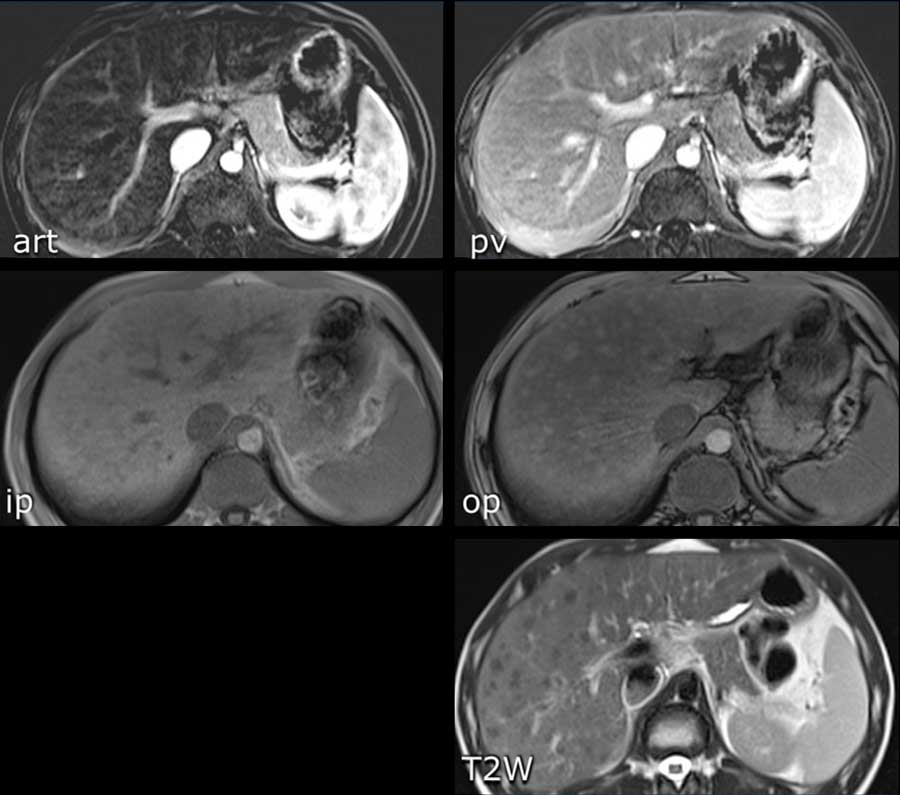
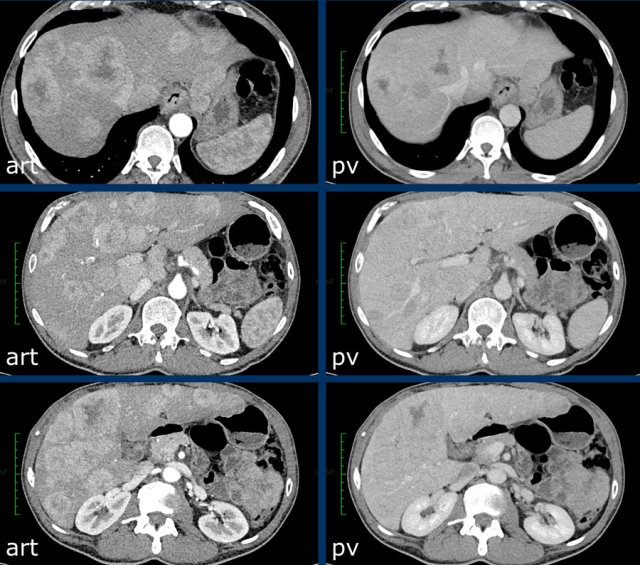
The CT-images show a lesion with enhancement that follows the bloodpool, which is typical for a hemangioma.
Continue with the MR of this patient.
MRI-images of the same patient.
The enhancement of this lesion follows the enhancement of the blood pool.
LI-RADS 3 - intermediate probability
LR-3 observations vary from benign lesions to dysplastic nodules to HCC.
Many LR-3s are vascular pseudolesions.
Of all LR-3 lesions approximately 37% are HCC and 39% are malignant (1).
Lesions that are placed in category LI-RADS 3 are:
- Nodules with features of focal nodular hyperplasia or hepatic adenoma.
- Nodules of < 20mm without major features but with one or more ancillary findings of malignancy such as intralesional fat, T2 hyperintensity, diffusion restriction and HB phase hypointensity.
- Nodules above 20 mm and without major or ancillary features.
Study the MR-images. What are the findings?
How would you score LI-RADS.
The findings are:
- Post contrast imaging shows a lesion with non-peripheral hyperenhancement.
- lesion < 2cm
- No additional features.
The lesion was classified as LI-RADS 3.
LI-RADS 3
Here another small hyperenhancing lesion without any additional feature like washout, capsule or threshold growth.
This was also classified as LI-RADS 3.
LI-RADS 3
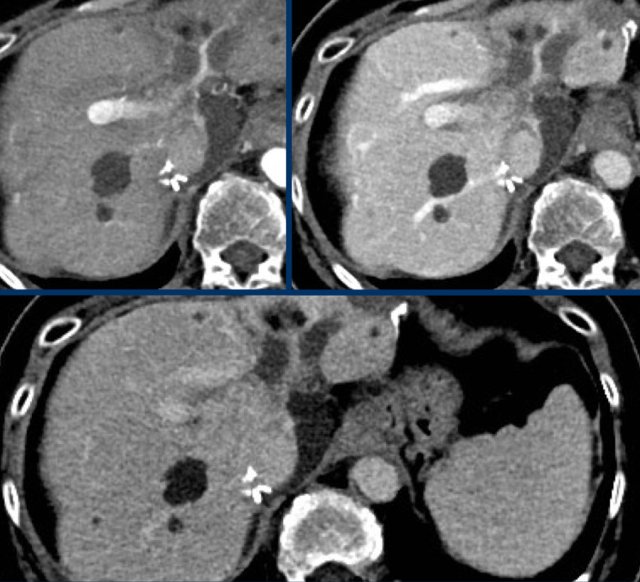
Arterial, PV and delayed phase images of a LIRADS 3 observation.
In segment 5 there is a subcapsular observation of intense arterial enhancement without washout in the PV or delayed phase.
LI-RADS 4 - probably HCC
Of all LR-4 observations about 74% are HCC and 81% are malignant (1).
The categorizing of an observation as LR-4 depends on the size of the lesion, the presence of APHE and the amount of additional major features (see LIRADS table).
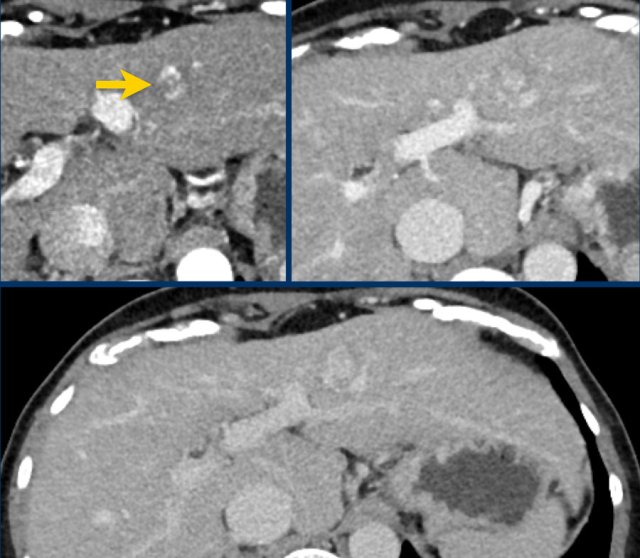
Here a very small lesion which measures less than 10 mm with nonrim arterial hyperenhancement and one additional feature, i.e. LI-RADS 4.
LI-RADS 5 - definitely HCC
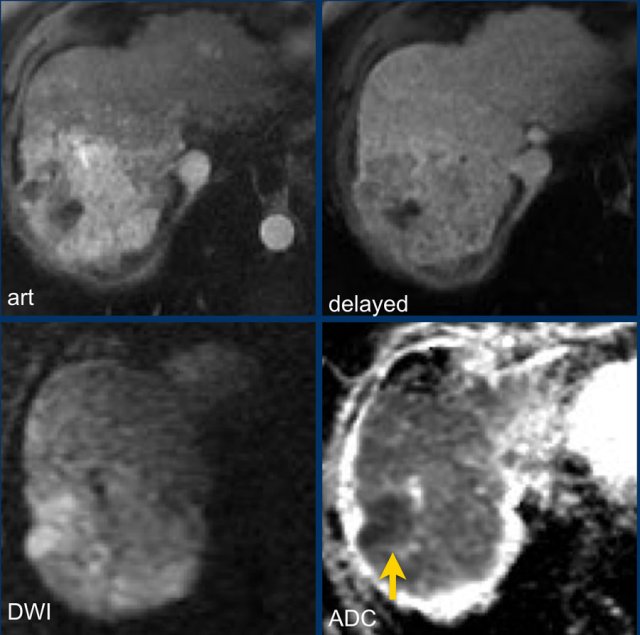
Study the MR-images.
What are the major findings and what are additional findings?
How would you score LI-RADS.
Major features - which makes this a LR-5 lesion:
- lesion > 20mm
- non-rim APHE
- washout
Ancillary features - which we will discuss in a moment:
- Diffusion restriction (arrow).
- Mosaic architecture - seen on delayed phase.
Here we did fill in the findings in the table:
- lesion > 20mm
- non-rim APHE
- washout
This is a LR-5 lesion.
Of all LR-5 lesions 95% are HCC and 98% are malignant.
In patients with concurrent extra-hepatic malignancy the positive predictive value of LR-5 for HCC drops, especially if the primary tumor is hypervascular.
When in doubt, categorizing a lesion as LR-M might be more appropriate in this group of patients.
LR-5
The images show an arterially enhancing lesion with washout in segment VI. suspicious of HCC: LR-5.
Note that the arterial enhancement is faint because the patient is scanned in the early arterial phase instead of the late arterial phase in which HCC has it’s peak enhancement.
Additional small lesions can therefore be easily missed.
LR-TIV - tumor in vein
The classification LR-TIV should be applied when there is unequivocal soft tissue within a vein, regardless if an associated mass is seen or not.
Almost always the venous invasion by tumor is related to a HCC.
LR-TIV is a contraindication to liver transplantation.
Malignancies other than HCC may also invade the portal venous system.
Additional clues of possible venous invasion by tumor are:
- Presence of an occluded vein with ill-defined margins
- Diffusion restriction
- Expansion of a vein
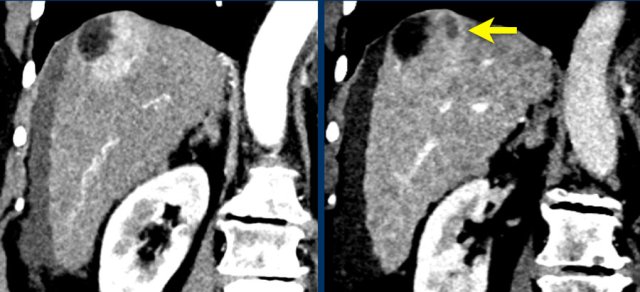
The images show an only mild rim-like arterially enhancing lesion in segment V with washout.
A linear area of hypoenhancement is seen extending from the mass which is suspicious of tumor in vein (yellow arrow).
Since we are not absolutely sure that it is a tumor thrombus, we cannot categorize this as LR-TIV.
A follow up CT was done, showing severe progression of the tumor as well as vascular involvement of the anterior right portal vein (white arrow).
Now we are sure of tumor invasion in the portal vein.
LR-M - malignant
The category of LR-M should be applied to malignant appearing lesions that do not have the typical characteristics of HCC.
Non-HCC malignant lesions either show:
- Targetoid appearance: rim-like APHE and peripheral washout and delayed central enhancement.
- Non-targetoid with an infiltrative growth pattern.
- Marked diffusion restriction or necrosis.
Categorizing a lesion as LR-M does not mean the diagnosis of HCC is excluded.
Of all LR-M lesions 2/3 are non-HCC malignancies like intrahepatic cholangiocarcinomas (CCA) or combined HCC-CCA and about 5% are benign.
This small percentage of benign malignant appearing lesions usually represent sclerosing hemangiomas or abscesses.
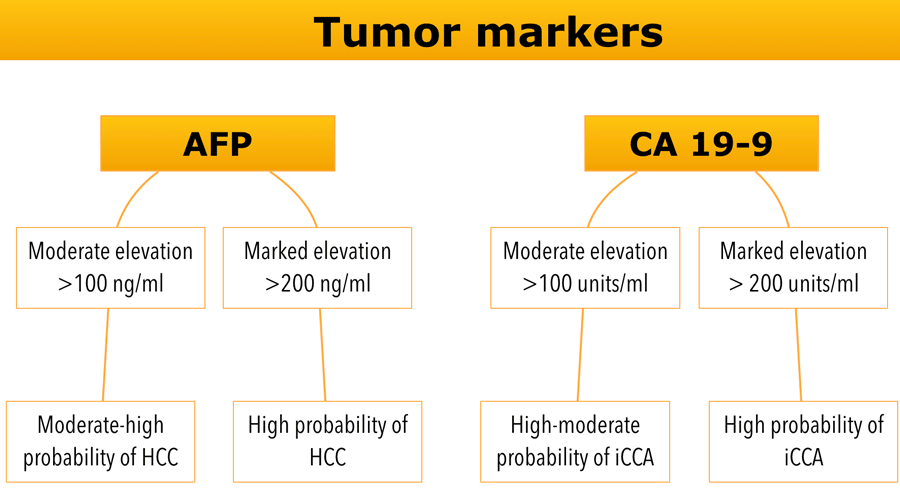
Tumor markers
Evaluation of circulating tumor biomarkers such as AFP and CA19-9 can be helpful to refine the differential diagnosis.
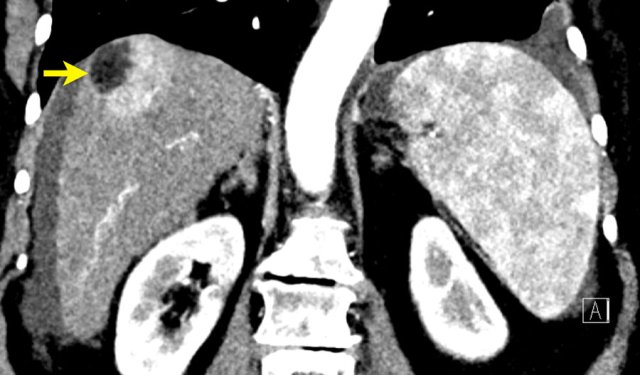
LR-M
The images show a large rim enhancing mass in the late arterial phase in a patient with chronic hepatitis.
In the portal venous phase there is progressive peripheral enhancement.
Pathology diagnosis confirmed this was not a HCC but a cholangiocarcinoma
LR-M
The images show a large, heterogeneous enhancing lesion in segment II.
There is peripheral enhancement in the portal venous phase. There is high signal on T1 IP and OOP imaging and diffusion restriction.
This lesion was resected and pathology showed a mixed HCC-iCCA.
Ancillary features
Ancillary features are findings that are helpful for detection improvement, increase in confidence for favoring the diagnosis HCC versus non-HCC malignancy or benignity or can lead to category adjustment.
These features are not obligatory and can be used at the radiologist’s discretion.
In case of category adjustment, observations can only be upgraded or downgraded one category.
However you are not allowed to upgrade from LR-4 to LR-5, because these ancillary features lack sufficient specificity for HCC to allow for an LR-5 upgrade.
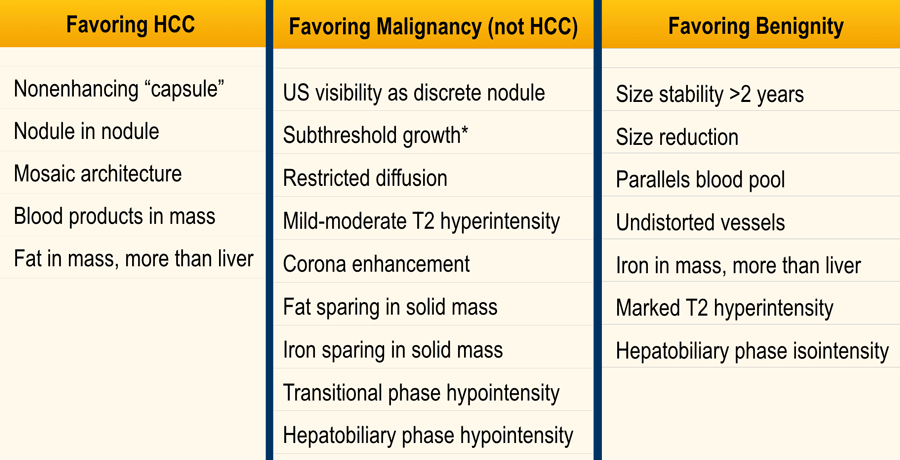
Features favoring HCC
Non-enhancing capsule is a feature of a capsule surrounding an observation not appearing as an enhancing rim.
Nodule in nodule is a nodule within a larger nodule with different imaging characteristics
Mosaic structure is randomly distributed compartments or nodules within an observation, usually with different imaging features.
Blood products in mass is intralesional or perilesional hemorrhage in the absence of biopsy or trauma.
Fat in mass is excess of fat within the whole or part of the mass, more than in adjacent liver. Can be large extracellular fat or intracellular.
Nodule in nodule
The images show a fat containing lesion with arterial hyperenhancement.
Within this lesion there is a nodule (arrow) with wash out and a capsule.
The lesion was classified as LR-5.
Mosaic architecture
The image shows an observation with hyperenhancement in the late arterial phase in a patient with cirrhosis.
Notice the mosaic architecture.
Blood products in mass
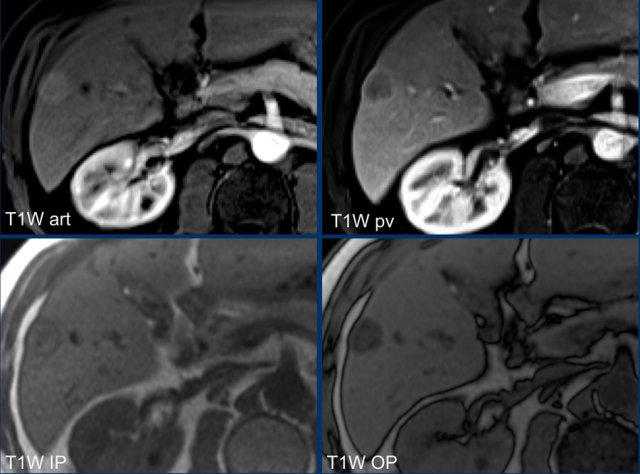
Study the MR-images.
What are the findings?
The findings are high signal in a mass in segment II both on an in-phase image as well as on the out-of-phase image (arrow).
This represents internal hemorrhage and in the absence of biopsy or trauma, is a feature that favors the diagnosis of HCC.
Fat in mass
Excess of fat in the whole or part of a mass is an ancillary finding that favors the diagnosis of HCC.
Same image as shown earlier.
What are the findings?
The findings are:
- Chirhosis: irregular contour of the liver, large spleen and ascites.
- Observation with non-rim enhancement and macroscopic fat in segment VII of the liver (-50 HU, yellow arrow).
This was classified as LI-RADS 5.
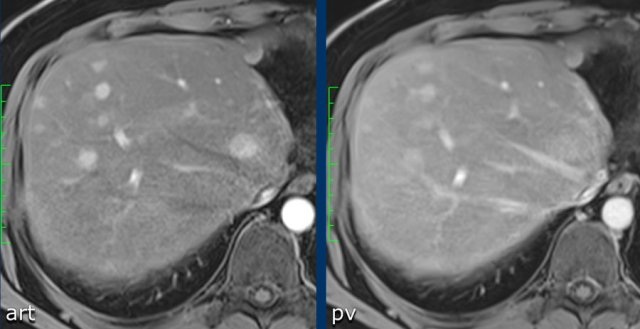
Study the MR-images.
What are the findings?
The findings are:
- non-rim APHE in segment VI of the liver
- Washout on PV phase
- Enhancing capsule
- Microscopic fat on IP/OOP sequences
Continue with next image...
Based on the findings on the images shown above, the lesion is classified as LI-RADS 5 because it is larger that 20mm and shows hyperenhancement and washout with a capsule.
The ancillary features favoring HCC are intracellular fat on IP/OOP imaging and diffusion restriction.
Now we can not go any higher than LI-RADS 5, but the additional features will give us extra confidence in the diagnosis.
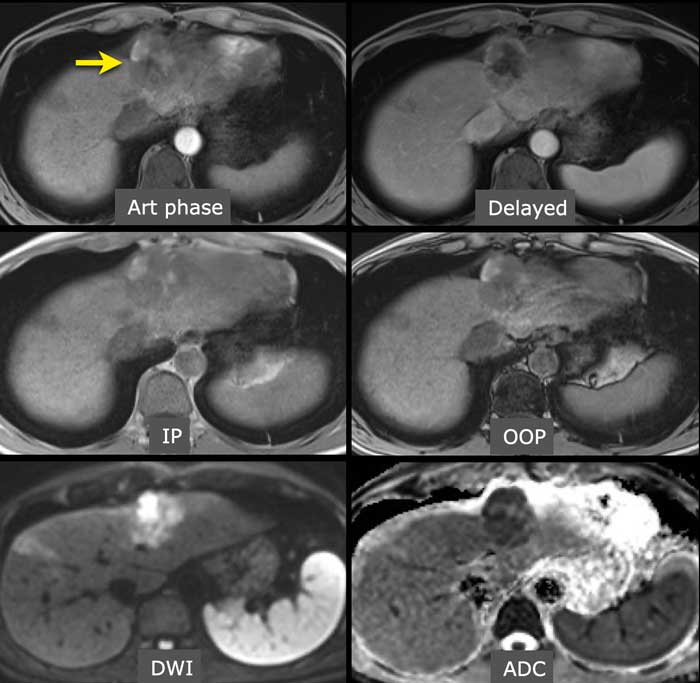
Diffusion restriction
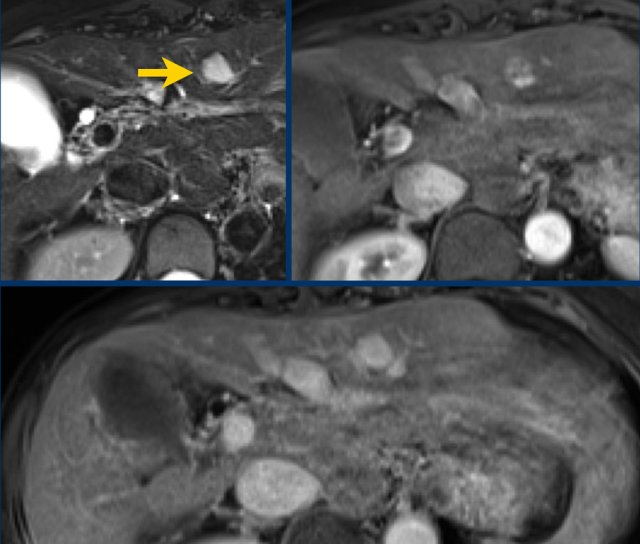
In this patient with cirrhosis the MR-images show an arterially enhancing observation (< 2 cm) in the right lobe (arrow).
As there is no washout or any other major feature observed this should be classified as a LR-3 lesion.
However due to the ancillary finding of diffusion restriction this observation can be upgraded to a LR-4.
This is a difficult topic, since diffusion restriction can also be seen in non-HCC tumors.
If there is doubt between the diagnosis of possible HCC and another type of malignancy a diagnostic biopsy can be considered.
Favoring non-HCC Malignancy
The table shows an overview of ancillary features favoring not HCC specific malignancy.
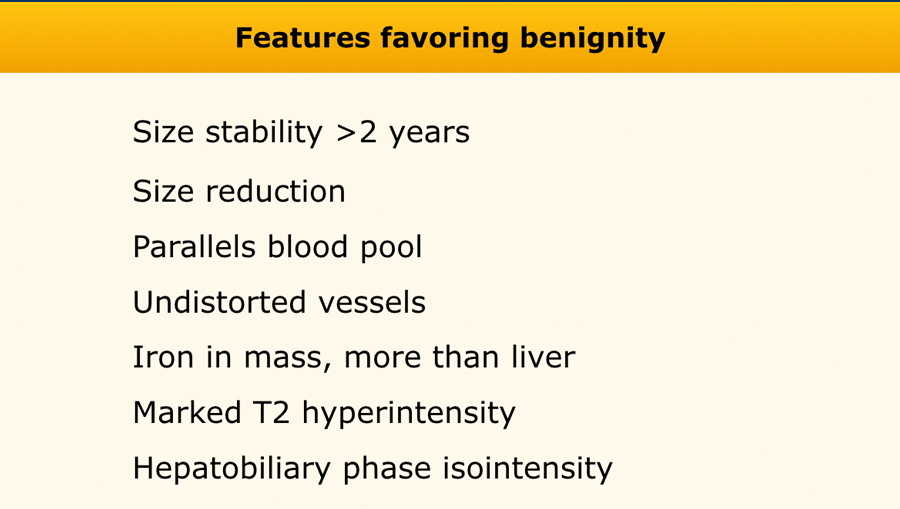
Features favoring benignity
The table shows an overview of benign ancillary features.
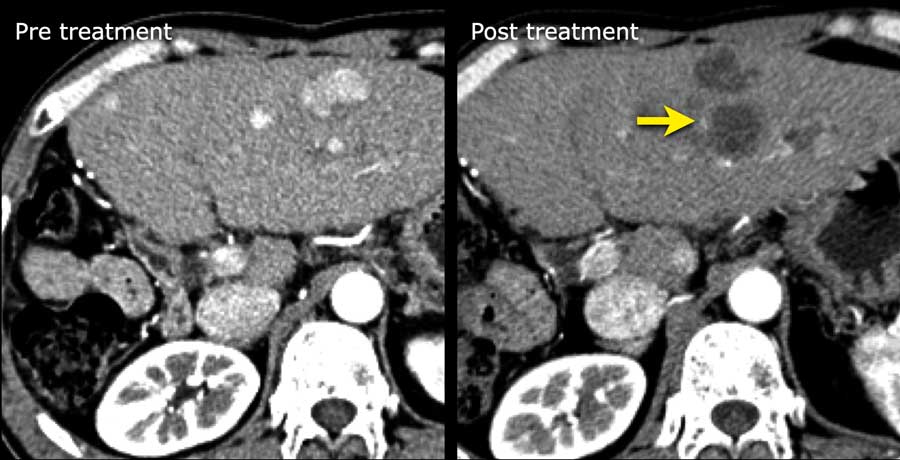
The images are of a patient with liver cirrhosis.
On the T2W-images there are multiple small nodules which are of low signal on the T2-weighted images.
This is due to the T2 shortening of iron.
This feature favors benignity.
There is no contrast enhancement of the lesions.
Management
LRTR - LI-RADS treatment response
Evaluation of treatment response
A different algorithm was created for the categorization of treated lesions.
Examples of locoregional therapies are radiofrequency ablation, percutaneous ethanol ablation, cryoablation, microwave ablation, transarterial embolization or chemoembolization, doxirubicin-eluted bead chemoembolization, transarterial radioembolization and external beam radiotherapy.
These lesions are either characterized as non evaluable, nonviable, equivocal or viable.
Treatment related parenchymal perfusional changes may mimic or obscure residual tumor, potentially leading to false positive or false negative assessment of viability.
If there is any uncertainty between two categories, the one reflecting lower certainty should be applied.
LI-RADS does not apply for systemic treatment.
LRTR non viable
If an observation shows no residual major or ancillary features or has completely disappeared it can be characterized as non-viable.
If typical post-treatment features are noted in the area of treatment and there are no signs of residual malignancy, a lesion can be considered as non viable.
The images show an ablation defect in segment V adjacent to gallbladder and hepatic vein without residual areas of arterial enhancement or washout.
This was classified as LRTR non viable.
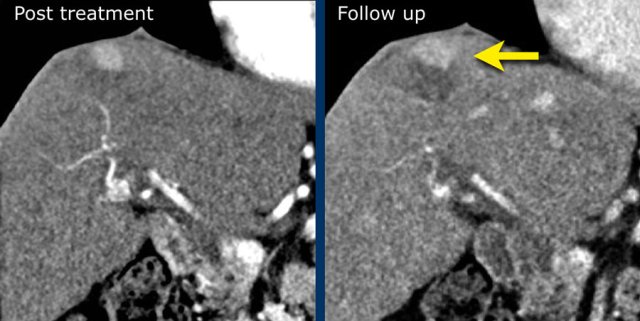
LRTR non-viable
The image in the late arterial phase show normal post treatment pattern with rim-like hyperenhancement of the surrounding liver parenchyma due to hyperemia after DEB-TACE (arrow).
DEB TACE is drug-eluting bead transarterial chemoembolization, a relative new endovascular treatment based on the use of microspheres to release chemotherapeutic agents within a target lesion with controlled pharmacokinetics.
DEB-TACE nowadays represents one of the most used treatments for unresectable hepatocellular carcinoma.
LRTR non-viable
Arterial enhancing lesions which were treated with Deb TACE. There is mild perilesional enhancement noted on the follow-up scan, which is a normal post-treatment finding.
LRTR equivocal
The enhancement pattern is atypical for treatment-specific expected enhancement and otherwise not meeting criteria for probably or definitely viable.
For some treatments early post-treatment enhancement patterns may not reliably differentiate viable from non viable tumor and therefore the most appropriate category in this early post-treatment period may be LRTR equivocal.
Area of arterial enhancement without washout in a segment 5 ablation defect (arrow), LRTR-equivocal.
LRTR viable
When measuring viable tumor only the largest continuous area of enhancement or washout should be measured (not traversing non enhancing area).
If residual enhancement within a lesion is nodular, the largest nodule should be reported.
LRTR viable
If a new tumor arises adjacent to a treated lesion this observation should be assigned a new non-treated LIRADS category.
If a new observation is noted at the surgical margin it should be assigned a category according to the treated lesion algorithm.
The coronal images shows a large lesion treated with TACE with residual areas of mild arterial enhancement and washout due to untreated supply via a phrenic artery (yellow arrow): LRTR-viable.
Continue with the axial image ...
Note there is irregularity of the peritoneal fat anteriorly due to capsular rupture (arrow).
LRTR viable
Previous segment II resection with viable tumor at the resection margin.
Note the area of arterial enhancement and washout adjacent to the surgical clips (arrows).
This is LRTR-viable.
Summary
Hypervascular metastases
Hypervascular metastases are sometimes difficult to differentiate from HCC because of their similar arterial enhancement pattern, especially when multiple lesions ae present.
Screening for HCC risk factors and patient history of primary tumors which can give hypervascular metastases (RCC, breast, neuroendocrine, melanoma, thyroid, choriocarcinoma, leiomyosarcoma) can be helpful in those cases.
Also absence of the typical HCC imaging characteristics should raise suspicion of another type of malignancy.
These are all images of a patient without cirrhosis, so we cannot use LI-RADS.
There are multiple heterogenous lesions with mild peripheral enhancement in the arterial phase.
Some lesions are mildly hypodense compared to adjacent liver parenchyma on the portal phase and larger lesions have a necrotic center.
Multiplicity and targetoid enhancement pattern are not typical for HCC and suggestive of metastatic disease.
This patient had a neuroendocrine tumor with liver metastases.
These images are of a patient with a steatotic liver.
Although some of these patient have a mildly greater chance of developing HCC, we cannot use LI-RADS.
There are multiple lesions which are hyperenhancing in the arterial and portal venous phase.
This is another example of hypervascular metastases.
This patient was known to have a neuroendocrine tumor of the pancreas.
Protocols
CT protocol
Required images for CT are late-arterial, portal venous and delayed phase.
Precontrast images are recommended after locoregional treatment.
Late arterial phase
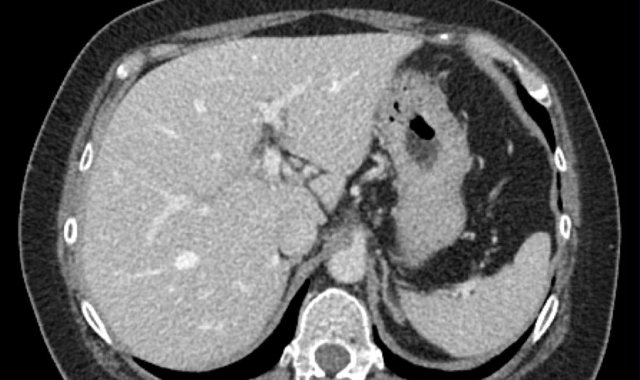
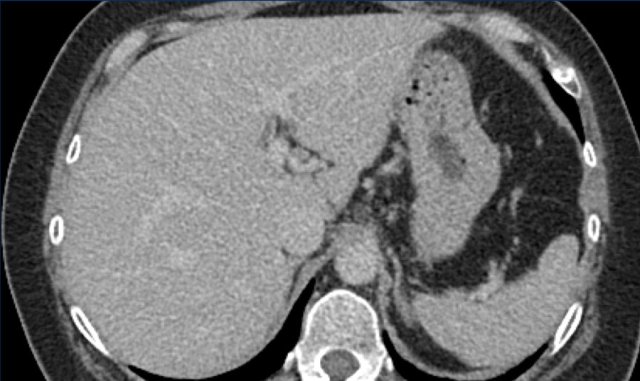
This phase refers to the hepatic arterial phase in which the hepatic artery and branches are fully enhanced and the hepatic veins are not yet enhancing.
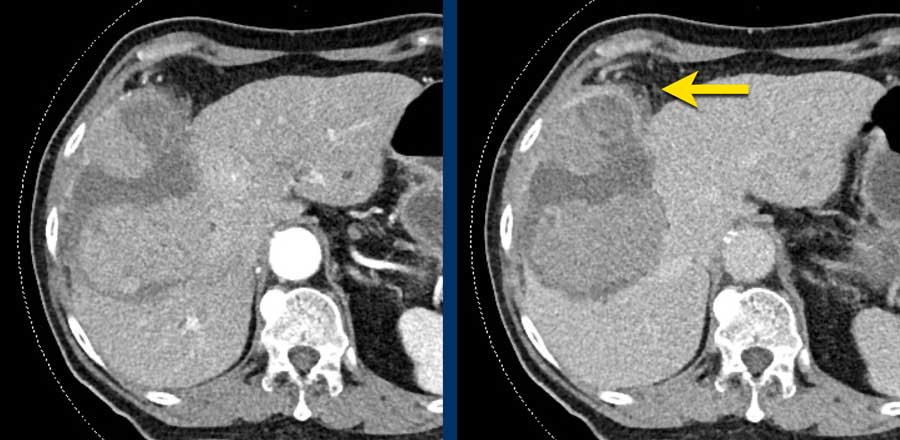
HCC usually enhances more strongly in the late arterial phase showing early enhancement of the portal vein and is therefore preferred over the early arterial phase, when there is no enhancement of PV and poor enhancement of the HCC.
Notice poor enhancement of liver and portal vein (white arrow) in the early arterial phase and good enhancement of both hepatic artery (white arrow) and portal vein (green arrow) in the late arterial phase.
Portal venous phase
There is complete enhancement of the portal veins with antegrade filling of hepatic veins.
In this phase the normal liver parenchyma is usually at its peak of enhancement.
Hypovascular lesions like most metastases are best detected in this phase, but hypervascular lesions are poorly seen because there is poor contrast between the good enhancement of the liver parenchyma and the hypervascular lesion.
Delayed phase
Portal and hepatic veins are enhanced but less than in the portal venous phase.
Liver parenchyma is enhanced but also less than in PV phase.
This phase is typically acquired 2-5 min after injection.
This phase is helpful in detecting wash out of HCC and in detecting sclerotic lesions like cholangiocarcinoma or sclerotic metastases like sometimes seen in breast cancer.
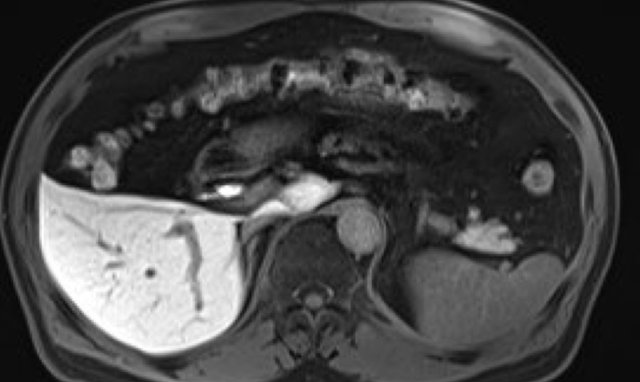 Hepatobiliary phase study: diffuse uptake of contrast by normal liver parenchyma with hypointense appearance of the vessels and contrast excreted within the bile ducts.
Hepatobiliary phase study: diffuse uptake of contrast by normal liver parenchyma with hypointense appearance of the vessels and contrast excreted within the bile ducts.
MRI protocol
The required sequences for MRI are:
- Unenhanced T1 IP and OP imaging
- T2 (with or without fat suppression)
- Multiphase T1 pre and post contrast.
- Optional sequences: diffusion-weighted and subtraction imaging.
Transitional phase
This phase is acquired with a hepatobiliary contrast agent after the extracellular phase but before the hepatobiliary phase.
Liver vessels and hepatic parenchyma are of similar intensity. Typically acquired 2-5 min after injection.
Hepatobiliary phase
This phase is acquired with a hepatobiliary agent.
Liver parenchyma is hyperintense to the hepatic vessels. There is excretion of contrast in the biliary system.
Typically acquired about 20 min after injection of gadoxetate or 1-3 hours after gadobenate.